
The tendency of two species to be disproportionate can be determined by examining the?
A.Phase diagram
B.Volume VS Temperature graph
C.Frost Diagram
D.Heat Capacity VS temperature graph
Answer
524.1k+ views
Hint: The phase diagram represents physical changes of elements who are under different conditions in temperature and pressure. Heat capacity is a function of temperature. The phase diagram represents the limit stability of various phases at equilibrium with respect to temperature, composition, etc.
Complete step by step solution:
It is determined by examining the frost diagram of the oxidation state. These diagrams are also called oxidation state diagrams. This diagram is the plot of the oxidation state vs free energy of species. Properties of the oxidation state of various elements are represented in this diagram. Multiplying the number of electron transfers during oxidation state change to standard reduction potential for change occurred we get values to be plotted on the y-axis. A species that is located at the bottom of the diagram is said to be thermodynamically stable. Since species that are thermodynamically stable are located at the bottom as per oxidation-reduction point of view. A species can undergo disproportion if it is situated in the convex curve of the diagram. Species which are under standard condition whose pH is $0$ for acidic condition and $14$ for basic condition only their information can be obtained in the frost diagram. Strong oxidizing agents are located on the upper left side of the diagram. Reducing agents are located on the upper right side of the diagram. The thermodynamic stability of the species is determined by this diagram.
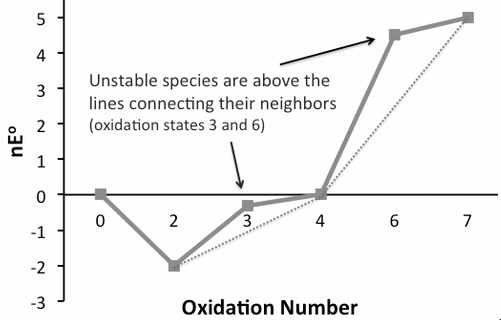
So, the option (C) frost diagram.
Note :
The relative stability of the species may vary on the change in pH. Some species are thermodynamically unstable towards reduction, they show very slow kinetics. Species on the concave curve do not disproportionate.
Complete step by step solution:
It is determined by examining the frost diagram of the oxidation state. These diagrams are also called oxidation state diagrams. This diagram is the plot of the oxidation state vs free energy of species. Properties of the oxidation state of various elements are represented in this diagram. Multiplying the number of electron transfers during oxidation state change to standard reduction potential for change occurred we get values to be plotted on the y-axis. A species that is located at the bottom of the diagram is said to be thermodynamically stable. Since species that are thermodynamically stable are located at the bottom as per oxidation-reduction point of view. A species can undergo disproportion if it is situated in the convex curve of the diagram. Species which are under standard condition whose pH is $0$ for acidic condition and $14$ for basic condition only their information can be obtained in the frost diagram. Strong oxidizing agents are located on the upper left side of the diagram. Reducing agents are located on the upper right side of the diagram. The thermodynamic stability of the species is determined by this diagram.

So, the option (C) frost diagram.
Note :
The relative stability of the species may vary on the change in pH. Some species are thermodynamically unstable towards reduction, they show very slow kinetics. Species on the concave curve do not disproportionate.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE

10 examples of friction in our daily life




