
The structure of TiCl is similar to CsCl. What would be the radius ratio in TiCl?
[A] 0.155 – 0.225
[B] 0.225 – 0.414
[C] 0.414 – 0.732
[D] 0.732 – 0.999
Answer
578.4k+ views
Hint: To solve this, you need to remember that CsCl has a coordination number of 8 and a body-centred cubic structure. Using the body diagonal of a cubic unit cell and the radius of the cations and the anions you can find the limiting value of the radius for a particular geometry.
Complete answer:
To answer this question, firstly let’s discuss radius ratio rule.
We know that an ionic solid consists of anions and cations arranged in a particular lattice arrangement. The radius ratio rule explains to us that with increase in the size of the cation, the number of anions of a particular size present around it will also increase. So, if we know the ratio of cation and anion, we can easily predict the crystal geometry.
Now, in the given question it is given to us that TiCl has CsCl like structure.
So, firstly we should know that CsCl has eight atoms at the corner of the cube and 1 atom at the centre of the cube. We can easily understand that this structure is BCC i.e. body-centred cubic lattice.
So now, let us calculate the radius ratio for a BCC structure.
Let us consider that the edge length of the cube is ‘a’ and the radius of the cation is ${{r}_{+}}$ and that of anion is ${{r}_{-}}$ .
Now, we can write that a = 2${{r}_{-}}$ as we can understand from the diagram below.
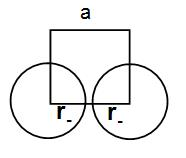
Similarly, if we consider the body diagonal we can write that –
${{r}_{-}}+2{{r}_{+}}+{{r}_{-}}=\sqrt{3}a$
Therefore, from the above two equations, we can write that –
$\begin{align}
& 2\left( {{r}_{+}}+{{r}_{-}} \right)=\sqrt{3}\left( 2{{r}_{-}} \right) \\
& Or,2{{r}_{+}}=2\sqrt{3}{{r}_{-}}-2{{r}_{-}} \\
& Or,2{{r}_{+}}=1.464{{r}_{-}} \\
& \frac{{{r}_{+}}}{{{r}_{-}}}=0.732 \\
\end{align}$
Therefore, we can write that the correct answer is option [D] 0.732 – 0.999.
Note:
In the discussion above, we have studied the radius ratio rule. Its application is in the prediction of crystal geometry. It also gives us an idea about thermal stability of carbonates, tendency of metal ions to form hydrated salts etc.
We can use this table to find the radius ratio for a particular co-ordination or vice versa-
Complete answer:
To answer this question, firstly let’s discuss radius ratio rule.
We know that an ionic solid consists of anions and cations arranged in a particular lattice arrangement. The radius ratio rule explains to us that with increase in the size of the cation, the number of anions of a particular size present around it will also increase. So, if we know the ratio of cation and anion, we can easily predict the crystal geometry.
Now, in the given question it is given to us that TiCl has CsCl like structure.
So, firstly we should know that CsCl has eight atoms at the corner of the cube and 1 atom at the centre of the cube. We can easily understand that this structure is BCC i.e. body-centred cubic lattice.
So now, let us calculate the radius ratio for a BCC structure.
Let us consider that the edge length of the cube is ‘a’ and the radius of the cation is ${{r}_{+}}$ and that of anion is ${{r}_{-}}$ .
Now, we can write that a = 2${{r}_{-}}$ as we can understand from the diagram below.

Similarly, if we consider the body diagonal we can write that –
${{r}_{-}}+2{{r}_{+}}+{{r}_{-}}=\sqrt{3}a$
Therefore, from the above two equations, we can write that –
$\begin{align}
& 2\left( {{r}_{+}}+{{r}_{-}} \right)=\sqrt{3}\left( 2{{r}_{-}} \right) \\
& Or,2{{r}_{+}}=2\sqrt{3}{{r}_{-}}-2{{r}_{-}} \\
& Or,2{{r}_{+}}=1.464{{r}_{-}} \\
& \frac{{{r}_{+}}}{{{r}_{-}}}=0.732 \\
\end{align}$
Therefore, we can write that the correct answer is option [D] 0.732 – 0.999.
Note:
In the discussion above, we have studied the radius ratio rule. Its application is in the prediction of crystal geometry. It also gives us an idea about thermal stability of carbonates, tendency of metal ions to form hydrated salts etc.
We can use this table to find the radius ratio for a particular co-ordination or vice versa-
| Limiting radius ratio of cation to anion | Structure | Co-ordination number |
| 0 - 0.155 | Linear | 2 |
| 0.155 - 0.225 | Trigonal planar | 3 |
| 0.225 - 0.414 | Tetrahedral | 4 |
| 0.414 - 0.732 | Octahedral | 6 |
| 0.414 – 0.732 | Square planar | 4 |
| 0.732 – 0.1 | Body centred cubic | 8 |
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




