
The structure of the alkane or cycloalkane with molecular formula \[{C_8}{H_{18}}\] that has only \[1^\circ \] H atoms is:
A.\[2,2,3,3 - \]tetramethyl butane
B.\[2,2,3 - \]trimethyl pentane
C.\[2,3,4 - \]trimethyl pentane
D.\[2,3,3 - \]trimethyl pentane
Answer
510.6k+ views
Hint: We need to know that the alkane is an organic compound and it is a cyclic or acyclic saturated hydrocarbon. And it contains carbon and hydrogen atoms and all the carbon - carbon bonds present in the alkane is single. The general chemical formula of alkane is \[{C_n}{H_{2n + 2}}\]. And the alkane is mainly divided in three types and that is, straight chain alkanes, branched alkanes and cycloalkanes.
Complete answer:
The structure of the alkane or cycloalkane with molecular formula \[{C_8}{H_{18}}\] that has only \[1^\circ \] H atoms is \[2,2,3,3 - \]tetramethyl butane. Here, the structure of \[2,2,3,3 - \]tetramethyl butane contains only primary hydrogen atoms. Let’s see the structure,
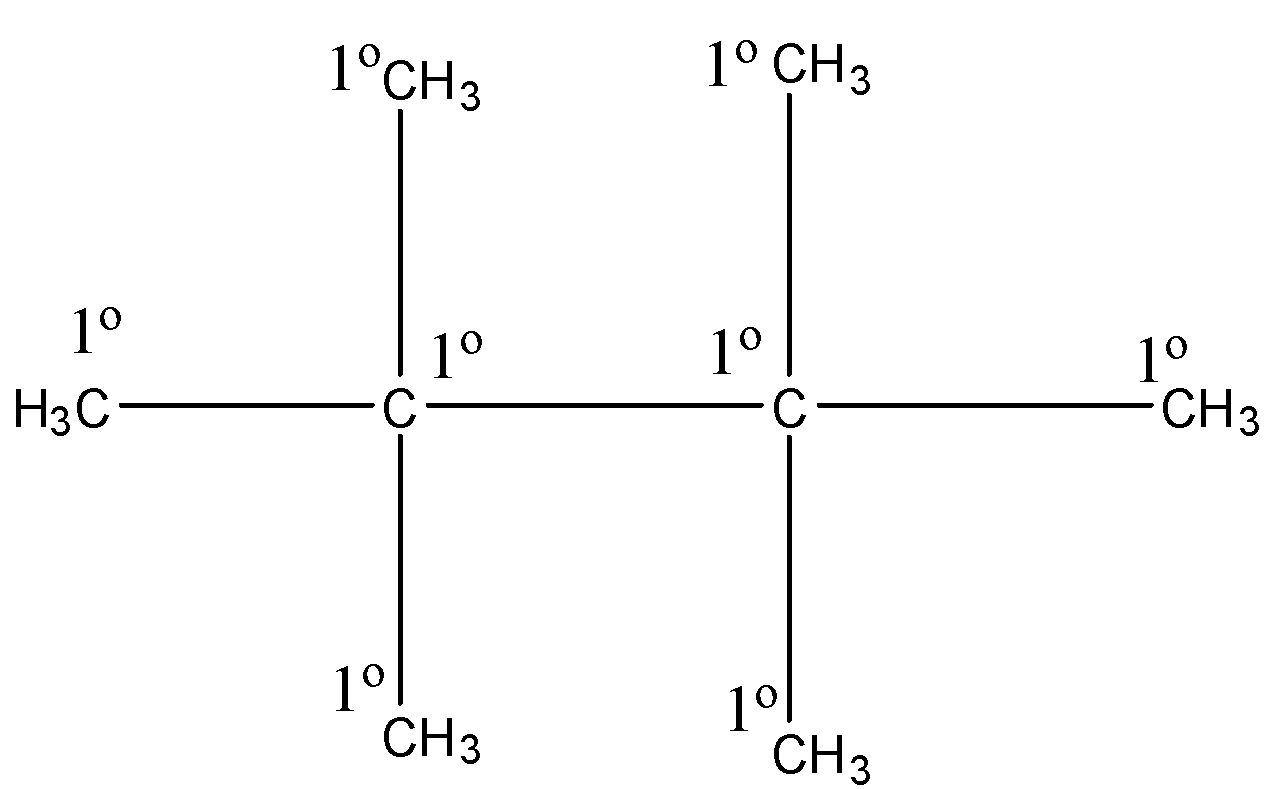
Hence, option (A) is correct.
In the case of Hence, \[2,2,3 - \]trimethyl pentane, all the hydrogen atoms are not primary. Because, it also presents secondary as well as tertiary hydrogen atoms. Hence, option (B) is incorrect.
In \[2,3,4 - \]trimethyl pentane, all the hydrogen atoms are not primary and it also contains both secondary and tertiary atoms. Hence, option (C) is incorrect.
The \[2,3,3 - \]trimethyl pentane not only contains primary hydrogen atoms. It also contains secondary, tertiary and quaternary atoms. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We need to know that in the case of primary hydrogen atoms, the hydrogen atom is occupied on a primary carbon atom in an organic compound. But in the case of secondary hydrogen atoms, it is attached to the secondary carbon atom. Similarly, the tertiary and quaternary hydrogen atoms are attached with tertiary and quaternary carbon atoms respectively.
Complete answer:
The structure of the alkane or cycloalkane with molecular formula \[{C_8}{H_{18}}\] that has only \[1^\circ \] H atoms is \[2,2,3,3 - \]tetramethyl butane. Here, the structure of \[2,2,3,3 - \]tetramethyl butane contains only primary hydrogen atoms. Let’s see the structure,

Hence, option (A) is correct.
In the case of Hence, \[2,2,3 - \]trimethyl pentane, all the hydrogen atoms are not primary. Because, it also presents secondary as well as tertiary hydrogen atoms. Hence, option (B) is incorrect.
In \[2,3,4 - \]trimethyl pentane, all the hydrogen atoms are not primary and it also contains both secondary and tertiary atoms. Hence, option (C) is incorrect.
The \[2,3,3 - \]trimethyl pentane not only contains primary hydrogen atoms. It also contains secondary, tertiary and quaternary atoms. Hence, the option (D) is incorrect.
Hence, option (A) is correct.
Note:
We need to know that in the case of primary hydrogen atoms, the hydrogen atom is occupied on a primary carbon atom in an organic compound. But in the case of secondary hydrogen atoms, it is attached to the secondary carbon atom. Similarly, the tertiary and quaternary hydrogen atoms are attached with tertiary and quaternary carbon atoms respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




