
The structure of isoprene is:
A. $C{{H}_{3}}-CH\equiv C=C{{H}_{2}}$
B. $C{{H}_{3}}-CH=C{{H}_{2}}$
C. ${{H}_{3}}C-C-C(C{{H}_{3}})=C{{H}_{2}}$
D. $C{{H}_{2}}=C(C{{H}_{3}})-C{{H}_{2}}-CH=C{{H}_{2}}$
Answer
569.4k+ views
Hint: Formula of isoprene is 2-methyl-1,3-butadiene. It is an organic compound which in pure form is a colourless volatile liquid.
Complete Solution :
- Isoprene is an unsaturated hydrocarbon which means it contains double bonds.
The prefix iso-, which stands for isomer, is usually given to 2-methyl alkanes. In other words, if there's methyl located on the second carbon of a carbon chain, we are able to use the prefix iso-. The prefix is placed before the alkane name that indicates the overall number of carbons.
- In short, we can describe the naming of the compounds written out with the substituents in alphabetical order followed by the bottom name (derived from the amount of carbons present within the parent chain). Commas are used between numbers and dashes are used between letters and numbers. There are not any spaces within the name.
- Now the IUPAC name of Isoprene is 2-methyl-1,3-butadiene.
So the parent chain contains 4 carbon atoms with one methyl group attached as substituent to the 2nd atom of the parent chain.
And also from the iupac name 2 double bonds are present at 1st and 3rd carbon of the parent chain.
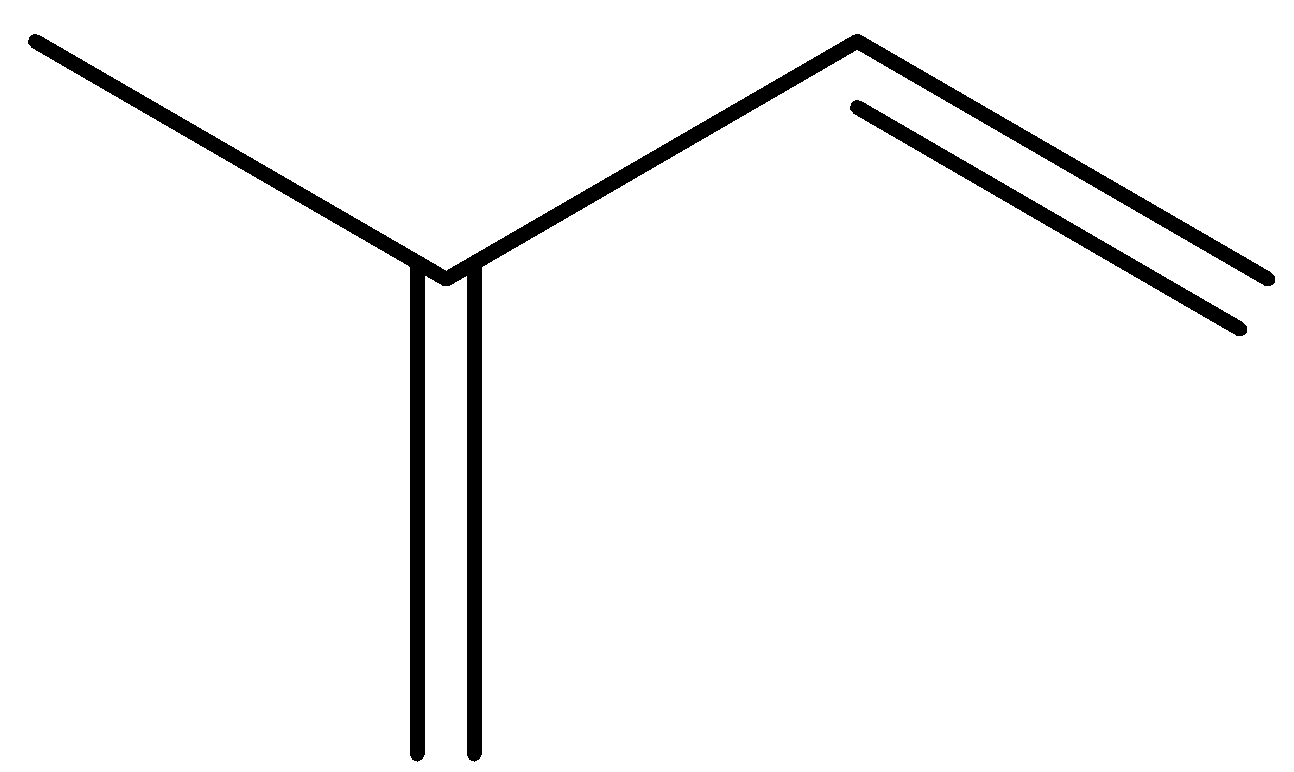
So comparing with the above options it matches with C .
So, the correct answer is “Option C”.
Note: Isoprene is used for processing petroleum or coal tar and also used as a chemical raw material.
- Polyisoprene, polymer of isoprene (${{C}_{5}}{{H}_{8}}$) that's the first chemical constituent of natural rubber, of the present resins balata and gutta-percha, and of the synthetic equivalents of those materials.
Complete Solution :
- Isoprene is an unsaturated hydrocarbon which means it contains double bonds.
The prefix iso-, which stands for isomer, is usually given to 2-methyl alkanes. In other words, if there's methyl located on the second carbon of a carbon chain, we are able to use the prefix iso-. The prefix is placed before the alkane name that indicates the overall number of carbons.
- In short, we can describe the naming of the compounds written out with the substituents in alphabetical order followed by the bottom name (derived from the amount of carbons present within the parent chain). Commas are used between numbers and dashes are used between letters and numbers. There are not any spaces within the name.
- Now the IUPAC name of Isoprene is 2-methyl-1,3-butadiene.
So the parent chain contains 4 carbon atoms with one methyl group attached as substituent to the 2nd atom of the parent chain.
And also from the iupac name 2 double bonds are present at 1st and 3rd carbon of the parent chain.

So comparing with the above options it matches with C .
So, the correct answer is “Option C”.
Note: Isoprene is used for processing petroleum or coal tar and also used as a chemical raw material.
- Polyisoprene, polymer of isoprene (${{C}_{5}}{{H}_{8}}$) that's the first chemical constituent of natural rubber, of the present resins balata and gutta-percha, and of the synthetic equivalents of those materials.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




