
The state of hybridization of boron and oxygen atom in boric acid $\left( {{H_3}B{O_3}} \right)$ is respectively:
A.$s{p^3},s{p^3}$
B.$s{p^2},s{p^3}$
C.$s{p^3},s{p^2}$
D.$s{p^2},s{p^2}$
Answer
588k+ views
Hint: We can determine the hybridization of an atom in a molecule, using steric numbers. We can calculate the steric number by summing up the number of atoms bonded to the central atom and the lone pairs of electrons on the central metal atom. Let us know that if the steric number is 4, then we say the atom is in $s{p^3}$ hybridization, if the steric number is 3, then it is $s{p^2}$ hybridization, if the steric number is 2, then it is $sp$ hybridization.
Complete step by step answer:We know the formula of boric acid is ${H_3}B{O_3}.$ we can draw the structure of boric acid as,
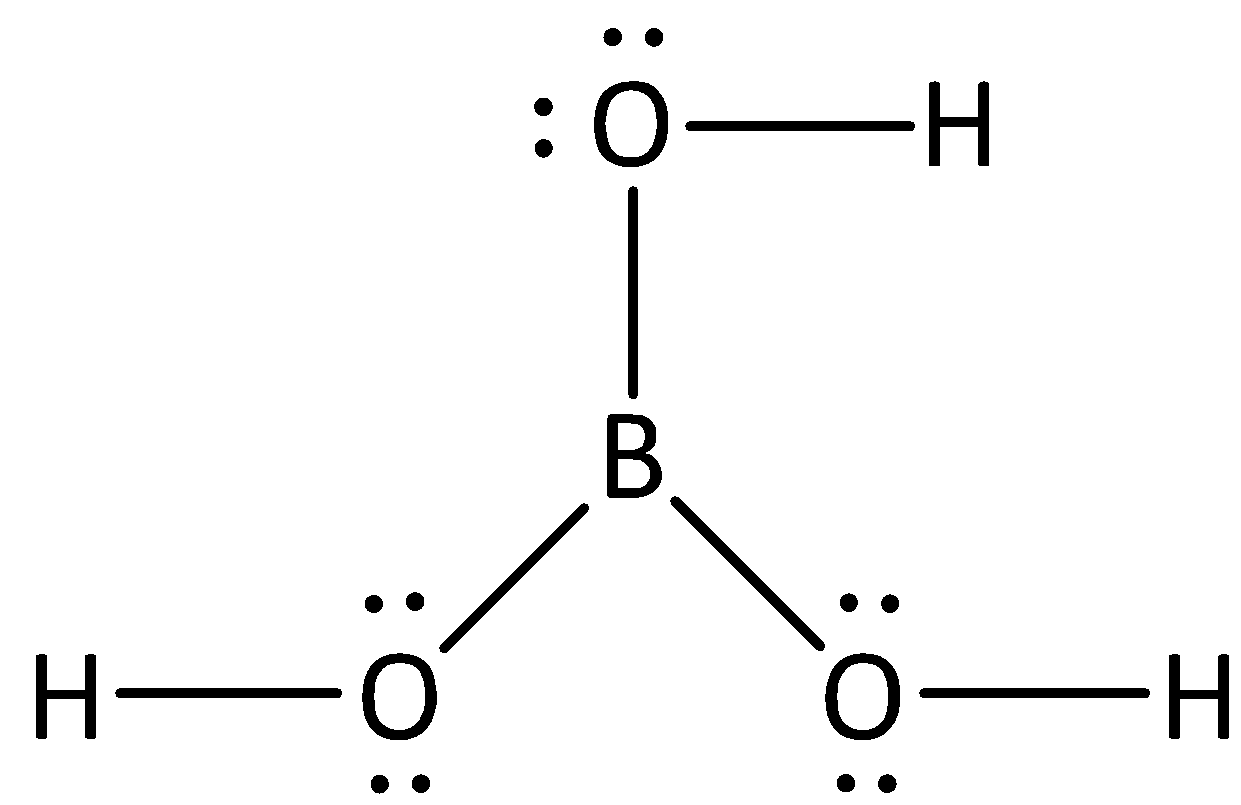
The steric number of boron atoms can be calculated by adding the number of atoms present in the central metal atom and lone pairs present in the central metal atom.
We can calculate the steric number of boron as,
\[
{\text{Steric number = No of atoms bonded to central atom + No of lone pairs in the central metal atom}} \\
{\text{Steric number = 3 + 0}} \\
{\text{Steric number = 3}} \\
\]
We have calculated the steric number of boron is 3. If an atom has steric number 3, we have to know that hybridization of atom will be $s{p^2}$.
Therefore, the hybridization of boron boric acid is $s{p^2}$.
In the structure of boric acid, we can see that the oxygen atoms consists of two bond pairs and two lone pairs, therefore the total number of groups present in oxygen is four, and if the number of groups present is four, then the hybridization of atom will be $s{p^3}$.
Therefore, the hybridization of oxygen in boric acid is$s{p^3}$.
Boric acid contains a network complex structure, and the geometry of boron is trigonal planar and is $s{p^2}$ hybridized whereas the geometry of oxygen is tetrahedral and has $s{p^3}$ hybridization with two lone pairs of electrons present in each atom of oxygen.
$\therefore $Option B is correct.
Note:
We can also determine the hybridization for organic molecules in the following method,
-If an atom contains single bonds, then it is $s{p^3}$ hybridized. Example for $s{p^3}$ hybridized molecule is methane. Generally, alkanes come under $s{p^3}$ hybridization. Molecules that have $s{p^3}$ hybridization will have tetrahedral geometry.
-If an atom contains double bonds, then it is $s{p^2}$ hybridized. Example for $s{p^2}$ hybridized molecule is ethene. Generally, alkenes come under $s{p^2}$ hybridization. Molecules that have $s{p^2}$ hybridization will have trigonal planar geometry.
-If an atom contains triple bonds, then it is $sp$ hybridized. Example for $sp$ hybridized molecule is ethyne. Generally, alkynes come under $sp$ hybridization.
Complete step by step answer:We know the formula of boric acid is ${H_3}B{O_3}.$ we can draw the structure of boric acid as,

The steric number of boron atoms can be calculated by adding the number of atoms present in the central metal atom and lone pairs present in the central metal atom.
We can calculate the steric number of boron as,
\[
{\text{Steric number = No of atoms bonded to central atom + No of lone pairs in the central metal atom}} \\
{\text{Steric number = 3 + 0}} \\
{\text{Steric number = 3}} \\
\]
We have calculated the steric number of boron is 3. If an atom has steric number 3, we have to know that hybridization of atom will be $s{p^2}$.
Therefore, the hybridization of boron boric acid is $s{p^2}$.
In the structure of boric acid, we can see that the oxygen atoms consists of two bond pairs and two lone pairs, therefore the total number of groups present in oxygen is four, and if the number of groups present is four, then the hybridization of atom will be $s{p^3}$.
Therefore, the hybridization of oxygen in boric acid is$s{p^3}$.
Boric acid contains a network complex structure, and the geometry of boron is trigonal planar and is $s{p^2}$ hybridized whereas the geometry of oxygen is tetrahedral and has $s{p^3}$ hybridization with two lone pairs of electrons present in each atom of oxygen.
$\therefore $Option B is correct.
Note:
We can also determine the hybridization for organic molecules in the following method,
-If an atom contains single bonds, then it is $s{p^3}$ hybridized. Example for $s{p^3}$ hybridized molecule is methane. Generally, alkanes come under $s{p^3}$ hybridization. Molecules that have $s{p^3}$ hybridization will have tetrahedral geometry.
-If an atom contains double bonds, then it is $s{p^2}$ hybridized. Example for $s{p^2}$ hybridized molecule is ethene. Generally, alkenes come under $s{p^2}$ hybridization. Molecules that have $s{p^2}$ hybridization will have trigonal planar geometry.
-If an atom contains triple bonds, then it is $sp$ hybridized. Example for $sp$ hybridized molecule is ethyne. Generally, alkynes come under $sp$ hybridization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




