
Which compound gives positive iodoform test?
A.2-pentanone
B.Pivaldehyde
C.Benzaldehyde
D.Isobutyl alcohol
Answer
531.1k+ views
Hint: Draw the molecular structure of each of the given options and remember positive iodoform test is given by compounds containing methyl ketones and 2-alkanols.

Complete step by step answer:
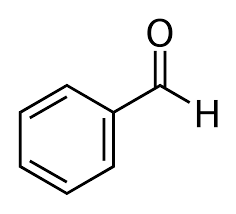
Option (c) Benzaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.
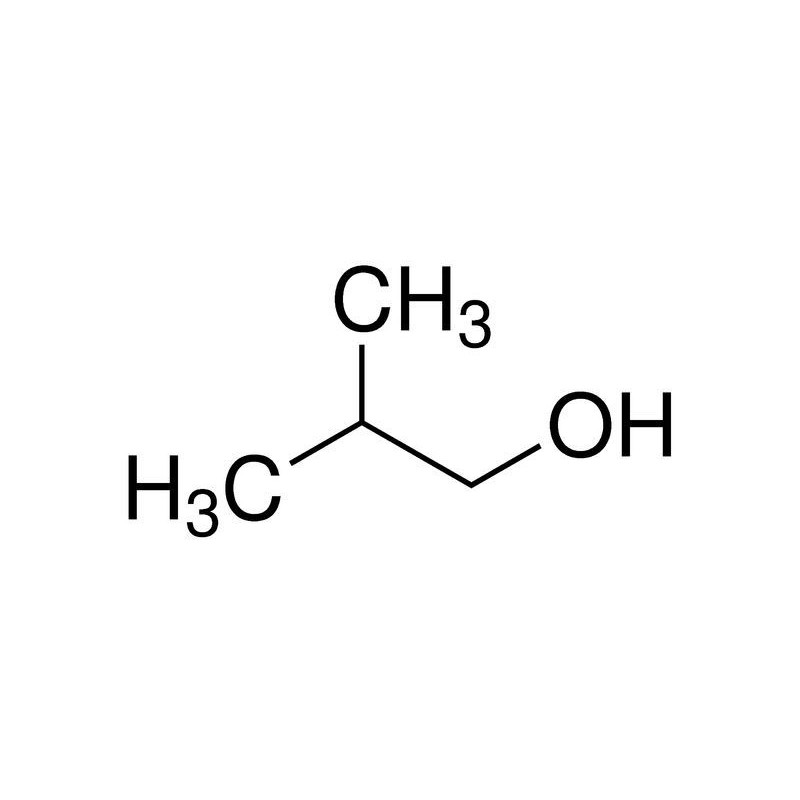
Option (d) Isobutyl alcohol does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.
So, correct option is A
Additional information: The pale yellow precipitate of iodoform ${ (CHI }_{ 3 }{ ) }$ is formed, which can be identified by its characteristic “antiseptic” smell.
Note: If an aldehyde gives a positive iodoform test, then it must be acetaldehyde since it is the only aldehyde with a ${ CH }_{ 3 }CO$ group.

Complete step by step answer:
Iodoform test is used for the identification of aldehyde and ketone.
A positive iodoform test is given by the compounds having ${ CH }_{ 3 }CO$ group in their structure.
When Iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or a secondary alcohol with a methyl group in the alpha position, a pale yellow precipitate of iodoform is formed.
Now we will look at the structure of each of the options provided.
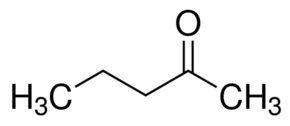
Option (a) 2-pentanone contains a methyl ketone group. Hence, it will give a positive iodoform test.

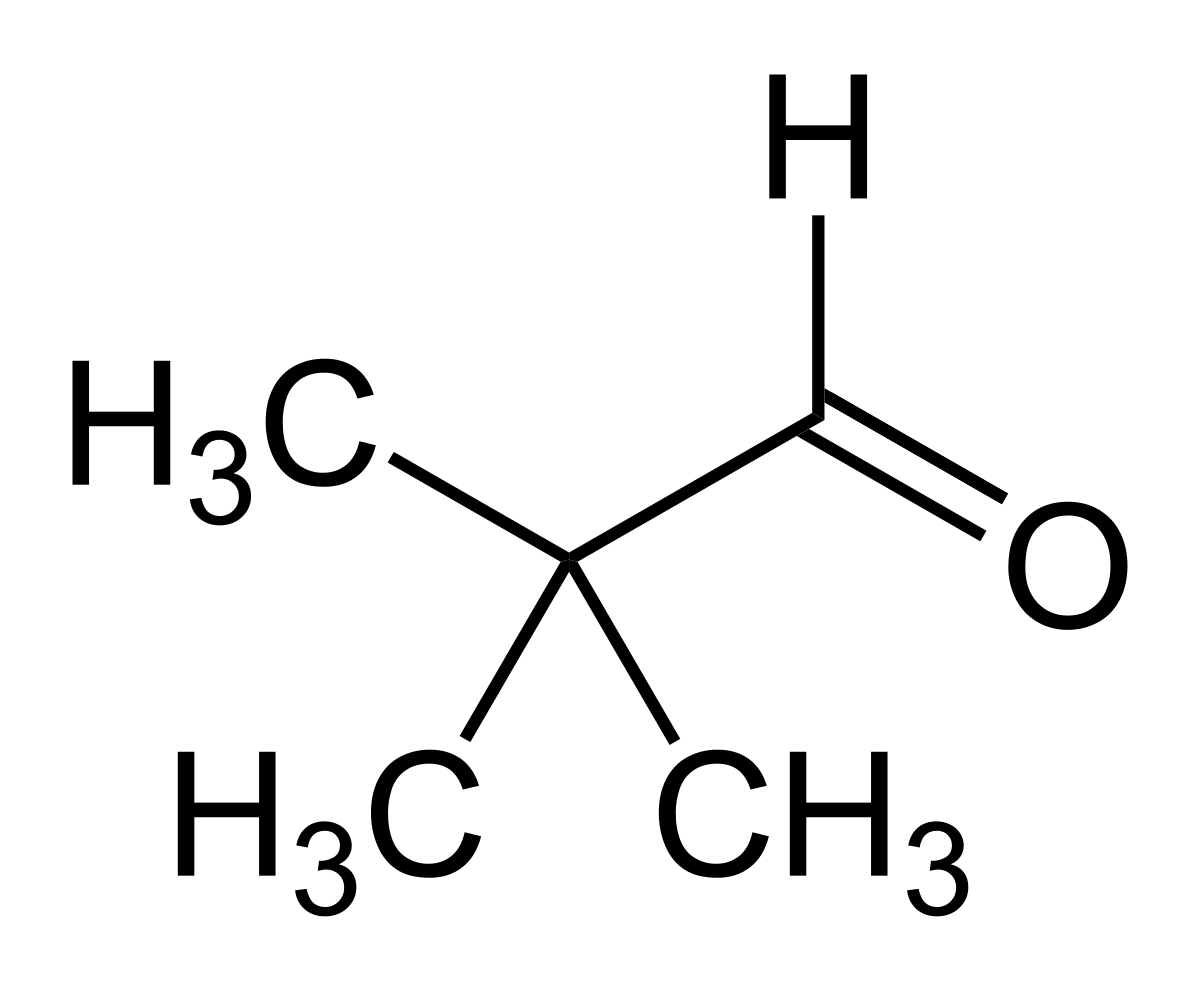
Option (b) Pivaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.

Option (c) Benzaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.

Option (d) Isobutyl alcohol does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.

So, correct option is A
Additional information: The pale yellow precipitate of iodoform ${ (CHI }_{ 3 }{ ) }$ is formed, which can be identified by its characteristic “antiseptic” smell.
Note: If an aldehyde gives a positive iodoform test, then it must be acetaldehyde since it is the only aldehyde with a ${ CH }_{ 3 }CO$ group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




