
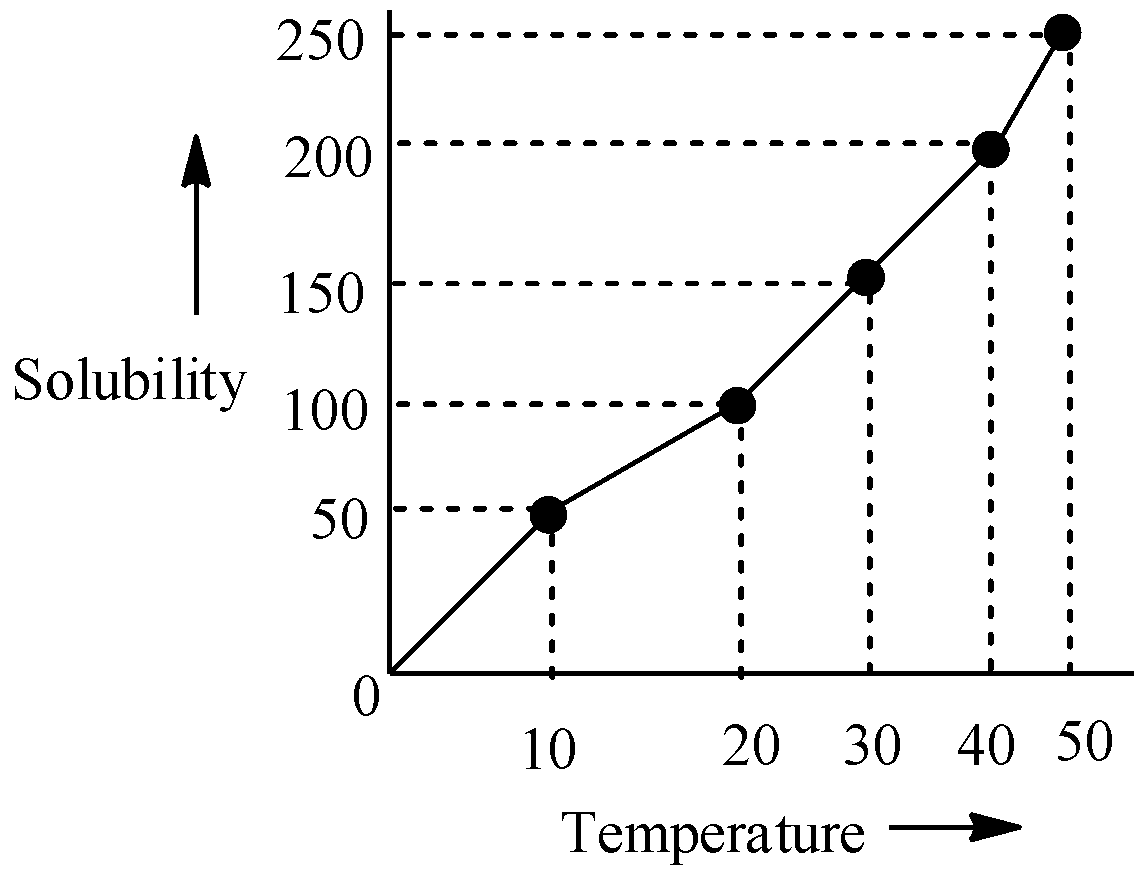
The solubility curve of $ KN{{O}_{3}} $ in water is given below. The amount of $ KN{{O}_{3}} $ that dissolves in 50g of water at $ {{40}^{0}}C $ is closest to:
A.100g
B. 150g
C. 200g
D. 50g

Answer
561k+ views
Hint Choose any specific point on the graph, preferably at $ {{40}^{0}}C $ and observe the solubility at that region. Use a unitary method to derive to a final conclusion.
Complete step by step solution:
In order to answer the question, we need to learn about solute, solvent and solutions. Now, in a mixture of two substances, one substance is present in low quantity and the other substance is present in more quantity. So, the substance that is present in smaller quantities is called the solute and the substance that is present in more quantities is called the solvent. In a binary mixture, both the solute and the solvent together is known as a solution.
Now, the solubility of the solute in a particular solvent depends upon many factors, one of them being the temperature. It is observed that by increasing the temperature, the solubility increases. For example, we can keep adding salt in 100mL of water but at one time no more salt will get dissolved in it. However, if we heat the water now, we can dissolve more salt in it. Now, if we cool down the water back to its normal temperature then the extra salt that was dissolved, will crystallize out. This is how temperature plays a role in the solubility.
Let us come to the question now. From the graph we can infer that as the temperature is increasing, the solubility is increasing too and it is more or less a straight line curve. We will pick up a specific point on the graph which is at $ {{40}^{0}}C $ . At this temperature, 200g of $ \,KN{{O}_{3}} $ can get dissolved in 100g of water. So, if we multiply the mass of water by $ \frac{1}{2} $ , then we can infer that 50g of water will dissolve $ (\frac{1}{2}\times 200)g $ of $ KN{{O}_{3}} $ , which is 100 grams, at $ {{40}^{0}}C $ .
So, we obtain the correct answer as option A.
NOTE: The reason for the increased solubility, on increasing the temperature accounts for the fact that as heat is gained by the solid the kinetic energy increases and facilitates the breaking of bonds that are held together by intermolecular attractions.
Complete step by step solution:
In order to answer the question, we need to learn about solute, solvent and solutions. Now, in a mixture of two substances, one substance is present in low quantity and the other substance is present in more quantity. So, the substance that is present in smaller quantities is called the solute and the substance that is present in more quantities is called the solvent. In a binary mixture, both the solute and the solvent together is known as a solution.
Now, the solubility of the solute in a particular solvent depends upon many factors, one of them being the temperature. It is observed that by increasing the temperature, the solubility increases. For example, we can keep adding salt in 100mL of water but at one time no more salt will get dissolved in it. However, if we heat the water now, we can dissolve more salt in it. Now, if we cool down the water back to its normal temperature then the extra salt that was dissolved, will crystallize out. This is how temperature plays a role in the solubility.
Let us come to the question now. From the graph we can infer that as the temperature is increasing, the solubility is increasing too and it is more or less a straight line curve. We will pick up a specific point on the graph which is at $ {{40}^{0}}C $ . At this temperature, 200g of $ \,KN{{O}_{3}} $ can get dissolved in 100g of water. So, if we multiply the mass of water by $ \frac{1}{2} $ , then we can infer that 50g of water will dissolve $ (\frac{1}{2}\times 200)g $ of $ KN{{O}_{3}} $ , which is 100 grams, at $ {{40}^{0}}C $ .
So, we obtain the correct answer as option A.
NOTE: The reason for the increased solubility, on increasing the temperature accounts for the fact that as heat is gained by the solid the kinetic energy increases and facilitates the breaking of bonds that are held together by intermolecular attractions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




