
The shape of s-orbital is:
Answer
508.8k+ views
Hint: We realize that there are electrons in an atom and that the electrons are often located in specific positions around the nucleus in various forms. S-orbital, p-orbital, d-orbital, and f-orbital are the four types of orbitals.
Complete answer:
We realize that there are many elements in existence, and they are classified according to the number of electrons in their atoms. We realize that there are electrons in an atom and that the electrons are often located in specific positions around the nucleus in various forms. As a result, this position is known as orbital. The shapes of orbitals and the number of electrons that can be accommodated in them distinguish various types of orbitals. S-orbital, p-orbital, d-orbital, and f-orbital are the four types of orbitals.
So, the s-orbital is an orbital in which the electrons can be found. Its shape is spherical, and all the charge density is within the shape. The maximum number of electrons that can be accommodated by any s-orbital is 2 and these must have opposite spin. There are many s-orbitals by the number of principal quantum numbers, i.e., 1s, 2s, 3s, 4s, 5s, 6s, and 7s. As the quantum number principle increases, the size of the s-orbital also increases.
The shape of s-orbital is given below-
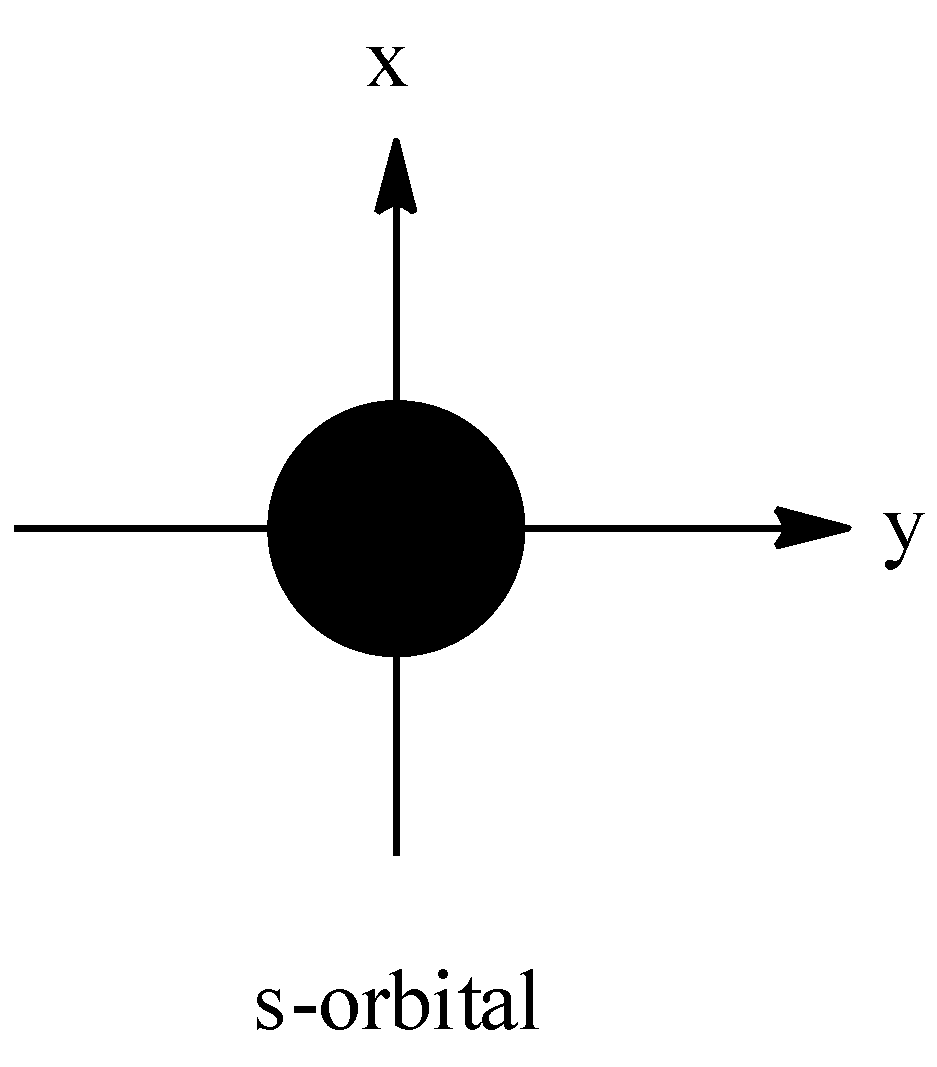
So, the shape of the s-orbital is spherical.
Note:
Other orbitals have different shapes, such as the p-orbital, which is dumb-bell shaped and can hold 6 electrons, the d-orbital, which is double dumb-bell shaped and can hold 10 electrons, and the f-orbital, which is tetrahedral shaped and can hold 14 electrons.
Complete answer:
We realize that there are many elements in existence, and they are classified according to the number of electrons in their atoms. We realize that there are electrons in an atom and that the electrons are often located in specific positions around the nucleus in various forms. As a result, this position is known as orbital. The shapes of orbitals and the number of electrons that can be accommodated in them distinguish various types of orbitals. S-orbital, p-orbital, d-orbital, and f-orbital are the four types of orbitals.
So, the s-orbital is an orbital in which the electrons can be found. Its shape is spherical, and all the charge density is within the shape. The maximum number of electrons that can be accommodated by any s-orbital is 2 and these must have opposite spin. There are many s-orbitals by the number of principal quantum numbers, i.e., 1s, 2s, 3s, 4s, 5s, 6s, and 7s. As the quantum number principle increases, the size of the s-orbital also increases.
The shape of s-orbital is given below-

So, the shape of the s-orbital is spherical.
Note:
Other orbitals have different shapes, such as the p-orbital, which is dumb-bell shaped and can hold 6 electrons, the d-orbital, which is double dumb-bell shaped and can hold 10 electrons, and the f-orbital, which is tetrahedral shaped and can hold 14 electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




