
The shape of s-orbital is _______ and the shape of p-orbital is _________.
A.Spherical, dumb-bell
B.Dumb-bell, spherical
C.Spherical, double dumb-bell
D.Spherical, spherical
Answer
543.3k+ views
Hint:To solve the above problem one must know the orbitals. There are four orbitals which are denoted as s-orbital, p-orbital, d-orbital, f-orbital. They all are based on the theory of quantum mechanics.
Complete step-by-step answer:First, we will understand the orbitals. The term ‘Atomic orbitals’ is defined as the mathematical function which represents the wave nature of electron in atoms.
There are four different orbitals of different sizes. Shape and orientation. The orbitals are denoted by as s-orbital, p-orbital, d-orbital, f-orbital. According to the quantum atomic model, the atom can have many possible numbers of orbitals.
Out of the four orbitals s, p, d, and f. The two orbitals s and p are the basic orbitals. The shape of orbitals is defined using the concept of the probability density of electrons. The shape of the orbital varies according to the probability of finding an electron.
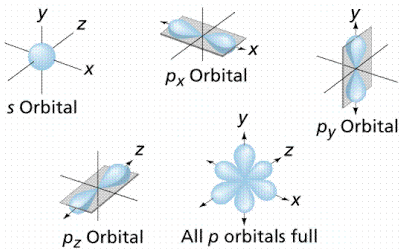
According to the quantum atomic model, the shape of the s-orbital looks like a sphere with a nucleus at its Centre. The shape of the s-orbital is symmetrical and therefore the probability density at a given distance equal in all directions.
The shape of the p-orbital is represented by lobes that lie on the plane passing through the nucleus. There are \[3\] p-orbitals that differ in orientation, shape, and size. The orbitals are \[2{p_x},2{p_y},2{p_z}\].
The \[3\]p-orbitals are represented as dumb-bell. The shape is represented as given below.
The shape of the s-orbital is Spherical and the shape of the p-orbital is dumb-bell.
Hence, the correct option is (A).
Note:The size of s-orbital and p-orbital increase with the increase in principal quantum number. The principal quantum number is represented by \[n\]. For example \[4s > 3s > 2s > 1s\] and \[4p > 3p > 2p\].
We can also predict the structures of s and p-orbital if we have the mathematical function of an atomic orbital.
Complete step-by-step answer:First, we will understand the orbitals. The term ‘Atomic orbitals’ is defined as the mathematical function which represents the wave nature of electron in atoms.
There are four different orbitals of different sizes. Shape and orientation. The orbitals are denoted by as s-orbital, p-orbital, d-orbital, f-orbital. According to the quantum atomic model, the atom can have many possible numbers of orbitals.
Out of the four orbitals s, p, d, and f. The two orbitals s and p are the basic orbitals. The shape of orbitals is defined using the concept of the probability density of electrons. The shape of the orbital varies according to the probability of finding an electron.
According to the quantum atomic model, the shape of the s-orbital looks like a sphere with a nucleus at its Centre. The shape of the s-orbital is symmetrical and therefore the probability density at a given distance equal in all directions.
The shape of the p-orbital is represented by lobes that lie on the plane passing through the nucleus. There are \[3\] p-orbitals that differ in orientation, shape, and size. The orbitals are \[2{p_x},2{p_y},2{p_z}\].
The \[3\]p-orbitals are represented as dumb-bell. The shape is represented as given below.
The shape of the s-orbital is Spherical and the shape of the p-orbital is dumb-bell.

Hence, the correct option is (A).
Note:The size of s-orbital and p-orbital increase with the increase in principal quantum number. The principal quantum number is represented by \[n\]. For example \[4s > 3s > 2s > 1s\] and \[4p > 3p > 2p\].
We can also predict the structures of s and p-orbital if we have the mathematical function of an atomic orbital.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




