
The shape of $I_3^ - $ is ----------------
A.tetrahedral
B.linear
C.T-shape
D.trigonal
Answer
595.2k+ views
Hint:We use VSEPR theory and Lewis theory to predict the shape and geometry of a compound. In this theory we use the number of bond and lone pairs of electrons to predict the geometry.
Complete step by step answer:
We know that I has the atomic number 53 and the electronic configuration of I is $\left[ {{\rm{Kr}}} \right]{\rm{4}}{{\rm{d}}^{{\rm{10}}}}{\rm{5}}{{\rm{s}}^{\rm{2}}}{\rm{5}}{{\rm{p}}^{\rm{5}}}$. The ${\rm{I}}_3^ - $molecule is formed by the bonding of the ${{\rm{I}}^ - }$with that of the ${{\rm{I}}_{\rm{2}}}$. The central
The atom gains one negative charge when the combination of the iodine atom takes place. In this compound, the ${{\rm{I}}^ - }$donates electron pairs and the ${{\rm{I}}_2}$molecule accepts the electrons. The electrons get accommodated inside the empty d-orbital. The ${\rm{I}}_3^ - $molecule is ${\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}$ hybridized and it has a linear geometry. This compound has a bond angle of ${\rm{10}}{{\rm{8}}^{\rm{0}}}{\rm{C}}$.
We can draw the structure of ${\rm{I}}_3^ - $ in the following way.
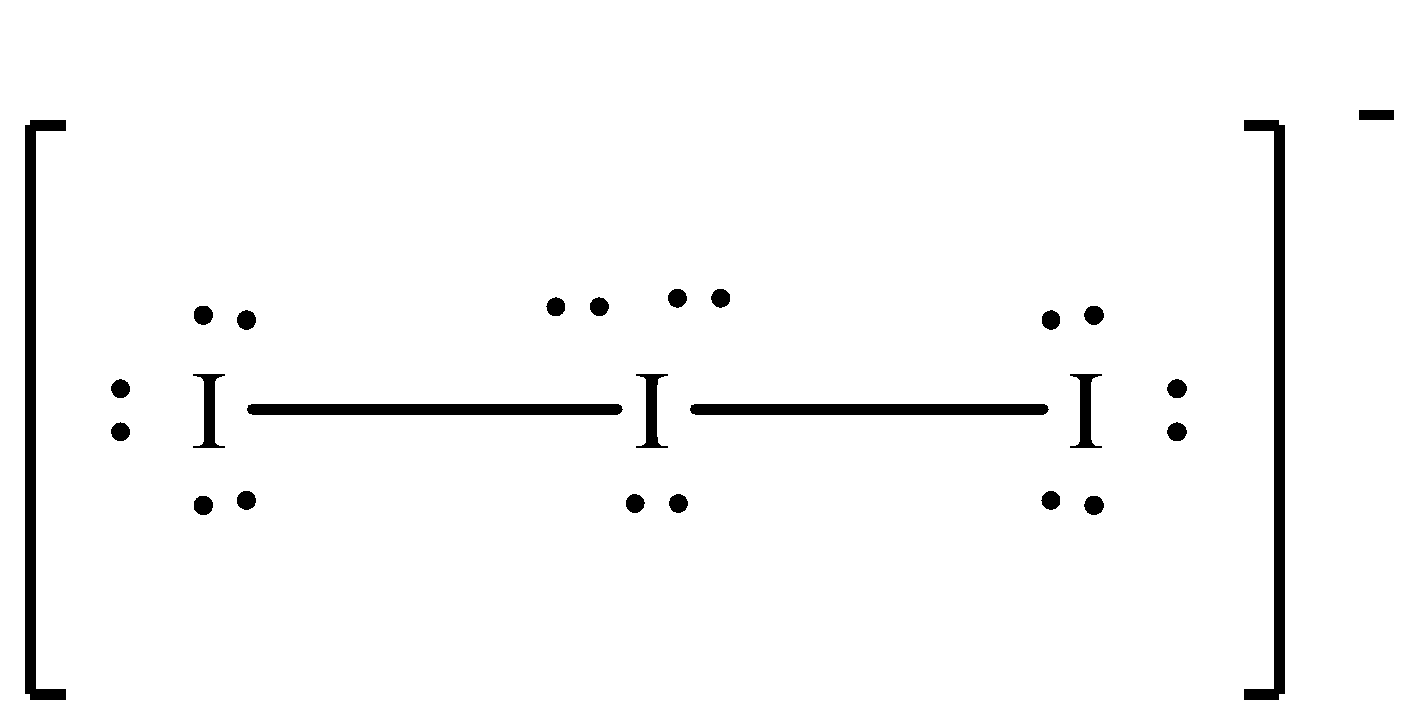
Therefore, out of the given four options, B is the correct option.
Additional information:
VSEPR helps in predicting the geometry of a compound taking in account the arrangement of electron pairs. VSEPR theory states that the electrons present around repel each other and it tends to take up an arrangement that will have minimum repulsion. The number of the valence shell present in the central metal atom is determined by drawing a Lewis structure of that atom. The number of electrons in the valence shell determines how and how many numbers of other atoms can find it and construct a structure.
Note:
VSEPR theory considers the repulsion by the lone pair to be much greater or effective than the repulsion by the bonding pair. Based on this theory, structures are classified as linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral etc.
Complete step by step answer:
We know that I has the atomic number 53 and the electronic configuration of I is $\left[ {{\rm{Kr}}} \right]{\rm{4}}{{\rm{d}}^{{\rm{10}}}}{\rm{5}}{{\rm{s}}^{\rm{2}}}{\rm{5}}{{\rm{p}}^{\rm{5}}}$. The ${\rm{I}}_3^ - $molecule is formed by the bonding of the ${{\rm{I}}^ - }$with that of the ${{\rm{I}}_{\rm{2}}}$. The central
The atom gains one negative charge when the combination of the iodine atom takes place. In this compound, the ${{\rm{I}}^ - }$donates electron pairs and the ${{\rm{I}}_2}$molecule accepts the electrons. The electrons get accommodated inside the empty d-orbital. The ${\rm{I}}_3^ - $molecule is ${\rm{s}}{{\rm{p}}^{\rm{3}}}{\rm{d}}$ hybridized and it has a linear geometry. This compound has a bond angle of ${\rm{10}}{{\rm{8}}^{\rm{0}}}{\rm{C}}$.
We can draw the structure of ${\rm{I}}_3^ - $ in the following way.

Therefore, out of the given four options, B is the correct option.
Additional information:
VSEPR helps in predicting the geometry of a compound taking in account the arrangement of electron pairs. VSEPR theory states that the electrons present around repel each other and it tends to take up an arrangement that will have minimum repulsion. The number of the valence shell present in the central metal atom is determined by drawing a Lewis structure of that atom. The number of electrons in the valence shell determines how and how many numbers of other atoms can find it and construct a structure.
Note:
VSEPR theory considers the repulsion by the lone pair to be much greater or effective than the repulsion by the bonding pair. Based on this theory, structures are classified as linear, trigonal planar, tetrahedral, trigonal bipyramidal, octahedral etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




