
The shape of ammonia molecule is:
A. Distorted tetrahedral
B. Hexahedral
C. Pentahedral
D. None of the above.
Answer
586.2k+ views
Hint: Shape of ammonia is predicted by valance cell electron pair (VSEPR) theory.In this molecule, 3 three hydrogen atoms are bonded and one unshared pair of electron is attached with the central nitrogen atom.
Complete step by step answer:
‘Nitrogen is the central atom of an ammonia molecule. Its electronic configuration is \[[He]\,\,2{s^2}\,2{p^3}\]so nitrogen has five valence electrons (outermost orbital electron).
Among them, three take point in covalent bond formation with three hydrogen atoms and the other two remain unshared as lone pairs. Lone pairs can’t decide the shape of molecules.
So, according to VSEPR theory, the shape of the ammonia molecule is distorted tetrahedral as there is no same type of four bonds.
An outline is shown by figure below.
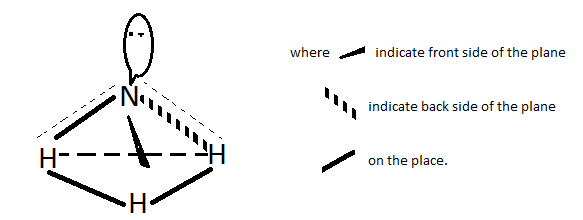
If an ammonia molecule had five bonds on six bonds then it would be Pentahedral or hexahedral.
So, the correct answer is “Option A”.
Note:
For geometry determination, we have to consider lone pair electron and bond pair electron both
So, for this case geometry of ammonia is tetrahedral and hybridization of central atom (here nitrogen) with be \[S{p^3}\], with an experimentally determined bond angle \[(H - N - H)\]of \[{106.7^o}\].
\['pe{l_3}'\]Molecule has some shape distorted tetrahedral.
Complete step by step answer:
‘Nitrogen is the central atom of an ammonia molecule. Its electronic configuration is \[[He]\,\,2{s^2}\,2{p^3}\]so nitrogen has five valence electrons (outermost orbital electron).
Among them, three take point in covalent bond formation with three hydrogen atoms and the other two remain unshared as lone pairs. Lone pairs can’t decide the shape of molecules.
So, according to VSEPR theory, the shape of the ammonia molecule is distorted tetrahedral as there is no same type of four bonds.
An outline is shown by figure below.

If an ammonia molecule had five bonds on six bonds then it would be Pentahedral or hexahedral.
So, the correct answer is “Option A”.
Note:
For geometry determination, we have to consider lone pair electron and bond pair electron both
So, for this case geometry of ammonia is tetrahedral and hybridization of central atom (here nitrogen) with be \[S{p^3}\], with an experimentally determined bond angle \[(H - N - H)\]of \[{106.7^o}\].
\['pe{l_3}'\]Molecule has some shape distorted tetrahedral.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




