
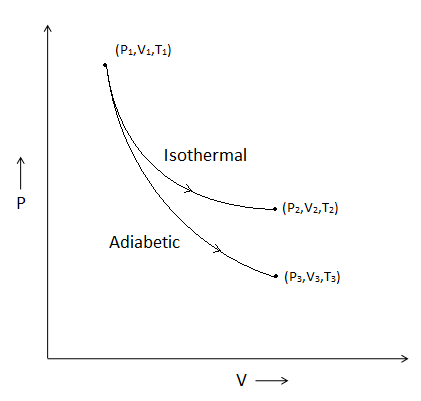
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is(are) correct?
[This question has multiple correct options]
(A) ${T_1} = {T_2}$
(B) ${T_3} > {T_1}$
(C) ${w_{isothermal}} > {w_{adiabatic}}$
(D) $\Delta {U_{isothermal}} > \Delta {U_{adiabatic}}$

Answer
588.3k+ views
Hint: Isothermal process is a process in which the temperature remains the same throughout the process. So, $\Delta T = 0$. In adiabatic processes, the heat of the system remains constant, so $\Delta Q = 0$ .
Complete answer:
We will find which of the statements are wrong as given in the graph.
- Isothermal process is a process in which the temperature remains the same throughout the process. In the isothermal curve, we can see that the initial temperature is ${T_1}$ and the final temperature is ${T_2}$. We know that as the process is isothermal, the temperature will not change. Thus, we can say that ${T_1} = {T_2}$.
- In adiabatic processes, the heat of the system remains constant. So, in this process, we can say that the initial temperature is shown as ${T_1}$ in the adiabatic curve. The final temperature is ${T_3}$. We can say that the final temperature is lower than the initial temperature from the curve. Thus, ${T_1} > {T_3}$.
- In the isothermal process, we know that $\Delta {U_{isothermal}}$ is zero. Thus, we can say that this energy gets converted into work (${w_{isothermal}}$) done. For adiabatic process, Q = 0. Thus, we can say that the work done by the isothermal process will be higher than the adiabatic process. So, ${w_{isothermal}} > {w_{adiabatic}}$
- We have seen earlier that the change in internal energy in isothermal energy is zero. Thus, $\Delta {U_{isothermal}} = 0$ . But in adiabatic processes, the work done is always negative. So, we can say that $\Delta {U_{isothermal}} > \Delta {U_{adiabatic}}$
Thus, we can conclude that options (A), (C) and (D) are correct.
Note:
Remember that a process can be called reversible if the change is brought in such a way that it can be reversed by a change. It involves equilibrium states in it. The processes that are not reversible are called irreversible processes.
Complete answer:
We will find which of the statements are wrong as given in the graph.
- Isothermal process is a process in which the temperature remains the same throughout the process. In the isothermal curve, we can see that the initial temperature is ${T_1}$ and the final temperature is ${T_2}$. We know that as the process is isothermal, the temperature will not change. Thus, we can say that ${T_1} = {T_2}$.
- In adiabatic processes, the heat of the system remains constant. So, in this process, we can say that the initial temperature is shown as ${T_1}$ in the adiabatic curve. The final temperature is ${T_3}$. We can say that the final temperature is lower than the initial temperature from the curve. Thus, ${T_1} > {T_3}$.
- In the isothermal process, we know that $\Delta {U_{isothermal}}$ is zero. Thus, we can say that this energy gets converted into work (${w_{isothermal}}$) done. For adiabatic process, Q = 0. Thus, we can say that the work done by the isothermal process will be higher than the adiabatic process. So, ${w_{isothermal}} > {w_{adiabatic}}$
- We have seen earlier that the change in internal energy in isothermal energy is zero. Thus, $\Delta {U_{isothermal}} = 0$ . But in adiabatic processes, the work done is always negative. So, we can say that $\Delta {U_{isothermal}} > \Delta {U_{adiabatic}}$
Thus, we can conclude that options (A), (C) and (D) are correct.
Note:
Remember that a process can be called reversible if the change is brought in such a way that it can be reversed by a change. It involves equilibrium states in it. The processes that are not reversible are called irreversible processes.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




