
What would be the resonating structure of $N{O_2}$?
Answer
546.3k+ views
Hint:As we know that according to the resonance concept, whenever a single lewis structure cannot describe the molecule accurately, a number of structures with similar energy, position of nuclei, bonding and antibonding pairs of electrons are taken as canonical structures of the hybrid which describes the molecule more accurately.
Complete step-by-step answer:It has been found that the experimentally observed properties of the certain compounds cannot be satisfactorily explained by writing a single lewis structure. So, a new concept of resonance was introduced to describe a molecule more accurately on the basis of its bonding in certain molecules. To do that a number of structures are taken into account which have the same energy, same position of nuclei, bonding and antibonding electron pairs which helps in more accurate information of the given molecule. The actual structure of the molecule is somewhere in between these hybrid structures.
We should know that the structures which are drawn to explain the properties of molecules are called its resonating structures and the actual structure is called the resonance hybrid.
Similarly, $N{O_2}$ possess a total of two resonating structures where nitrogen have an unpaired electron and a positive charge and oxygen possess a negative charge a number of unpaired electrons which we can show as below:
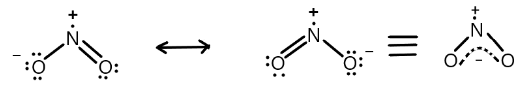
Note:Always remember that the resonance hybrids are always more stable than any one of its canonical forms. The stability of resonance hybrid is attributed to resonance energy which is equal to the difference in energy of resonance hybrid and its most stable canonical form. Also, the stability of the resonance hybrid increases with an increasing number of equivalent contributing resources.
Complete step-by-step answer:It has been found that the experimentally observed properties of the certain compounds cannot be satisfactorily explained by writing a single lewis structure. So, a new concept of resonance was introduced to describe a molecule more accurately on the basis of its bonding in certain molecules. To do that a number of structures are taken into account which have the same energy, same position of nuclei, bonding and antibonding electron pairs which helps in more accurate information of the given molecule. The actual structure of the molecule is somewhere in between these hybrid structures.
We should know that the structures which are drawn to explain the properties of molecules are called its resonating structures and the actual structure is called the resonance hybrid.
Similarly, $N{O_2}$ possess a total of two resonating structures where nitrogen have an unpaired electron and a positive charge and oxygen possess a negative charge a number of unpaired electrons which we can show as below:

Note:Always remember that the resonance hybrids are always more stable than any one of its canonical forms. The stability of resonance hybrid is attributed to resonance energy which is equal to the difference in energy of resonance hybrid and its most stable canonical form. Also, the stability of the resonance hybrid increases with an increasing number of equivalent contributing resources.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




