
The relationship between ($X$) and ($Y$) in this reaction is:
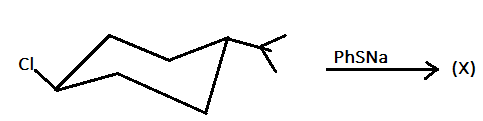
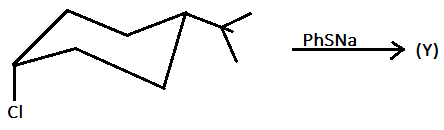
A.) constitutional isomer
B.) enantiomer
C.) identical
D.) none of these.


Answer
573.9k+ views
Hint: In this reaction, when $PhSNa$ reacts with the given chair structure then $PhSNa$ acts as a nucleophile and in the given compound the $PhSNa$ will substitute the chlorine attached in the compound but with inversion.
Complete step by step answer:
In the given reaction, the reagent $PhSNa$ has the chemical name as sodium thio-phenolate. When we react to thiophenol that is $PhSH$ with sodium hydroxide ($NaOH$) then the sodium thio-phenolate ($PhSNa$) is obtained.
When the sodium thio-phenolate ($PhSNa$) is reacted with the given compound then the sodium thio-phenolate ($PhSNa$) acts as a nucleophile and ${(PhS)^ - }$ will substitute the chlorine present in the compound and also the configuration of the ${(PhS)^ - }$ will get invert or in reverse position as that of chlorine. Therefore, both the above reactions can be written as:
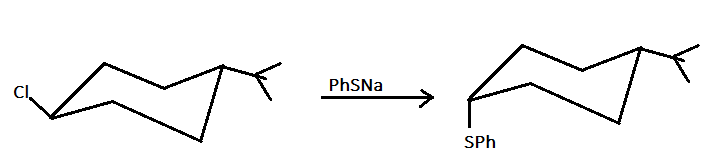
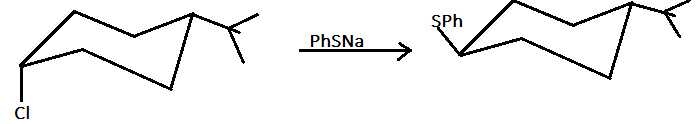
As we can see here, that compound $X$ and $Y$ have the same molecular formula but both are different in their connectivity. As in compound $X$ , the two functional groups are attached on the same side therefore compound $X$ is cis isomer and in compound $Y$, the two functional groups are attached on different sides therefore compound $Y$ is a trans isomer.
For option A.), The constitutional isomers are those isomers which have the same molecular formula but are different in their connectivity. The cis and trans isomers are constitutional isomers. Hence, compound $X$ and $Y$ are constitutional isomers.
For option B.), The enantiomers are mirror images of each other but the compound $X$ and $Y$ are not mirror images of each other.
For option C.), The identical compounds are those compounds which have the same molecular formula and structure also. But the compound $X$ and $Y$ are different in structure. Hence, they are not identical.
Hence, option A.) is the correct answer.
Note:
Always remember that if two functional groups are attached on the same side of carbon chain in a compound then it is called as cis isomer and if two functional groups are attached on the different sides of carbon in a compound then it is called as trans isomer.
Complete step by step answer:
In the given reaction, the reagent $PhSNa$ has the chemical name as sodium thio-phenolate. When we react to thiophenol that is $PhSH$ with sodium hydroxide ($NaOH$) then the sodium thio-phenolate ($PhSNa$) is obtained.
When the sodium thio-phenolate ($PhSNa$) is reacted with the given compound then the sodium thio-phenolate ($PhSNa$) acts as a nucleophile and ${(PhS)^ - }$ will substitute the chlorine present in the compound and also the configuration of the ${(PhS)^ - }$ will get invert or in reverse position as that of chlorine. Therefore, both the above reactions can be written as:


As we can see here, that compound $X$ and $Y$ have the same molecular formula but both are different in their connectivity. As in compound $X$ , the two functional groups are attached on the same side therefore compound $X$ is cis isomer and in compound $Y$, the two functional groups are attached on different sides therefore compound $Y$ is a trans isomer.
For option A.), The constitutional isomers are those isomers which have the same molecular formula but are different in their connectivity. The cis and trans isomers are constitutional isomers. Hence, compound $X$ and $Y$ are constitutional isomers.
For option B.), The enantiomers are mirror images of each other but the compound $X$ and $Y$ are not mirror images of each other.
For option C.), The identical compounds are those compounds which have the same molecular formula and structure also. But the compound $X$ and $Y$ are different in structure. Hence, they are not identical.
Hence, option A.) is the correct answer.
Note:
Always remember that if two functional groups are attached on the same side of carbon chain in a compound then it is called as cis isomer and if two functional groups are attached on the different sides of carbon in a compound then it is called as trans isomer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




