
The reduction of phenol to benzene is carried out with sodium borohydride.
A.True
B.False
Answer
581.7k+ views
Hint: Phenol is an organic compound that is made up of a phenyl group attached to an alcohol group. It has a sweet and tarry odor. It is used in the production of various chemicals. It shows acidic nature as its conjugate base is very stable due to resonance.
Complete step by step answer:
Phenols are cyclic organic compounds that are formed when one or more sites on a benzene ring, substitute their hydrogen atoms in exchange for the corresponding number of \[ - OH\] or alcoholic functional group.
Sodium borohydride is a weak reducing agent; it cannot reduce the electron-rich phenol to prepare benzene. But phenol can be converted into benzene by reaction with Zinc dust. the reaction is shown below,
\[{C_6}{H_5}OH\,\,\xrightarrow{{Zn\,\,Dust/\Delta }}{C_6}{H_6}\]
So, the given statement is false.
The correct option is B.
Additional information:
Now, phenols are formed as a product in various reactions, but it is not possible to completely extract all of the phenol formed from these reactions.
Hence, certain methods are developed just to maximize the extraction of phenol from a given chemical reaction. One such process is known as the Cumene process.
Cumene process for manufacturing phenol has many names including cumene – phenol process and Hock process. This method is used for producing both phenol and acetone. The reactants used in this reaction are benzene and propylene. Another important reactant required is oxygen, which is obtained from the air. Also, to be included is a small amount of radical initiators. This method is used all over the world for the production of phenol. The chemical reaction involved in this process is as follows:
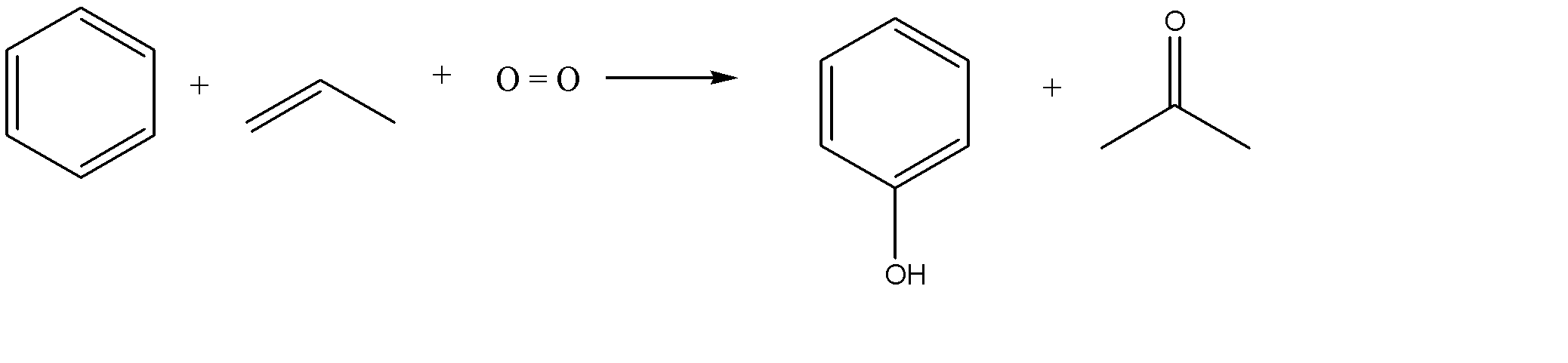
Note:The cumene process is a widely used method for the production of phenol. It involves synthesizing two relatively cheap compounds, viz. Benzene and propylene, into two extremely useful products like phenol and acetone. The most important chemical made from phenol is bisphenol A, which is used to make the polycarbonates. Phenol is also catalytically reduced to cyclohexanol which is used in the manufacture of polyamides 6 and 6, 6.
Complete step by step answer:
Phenols are cyclic organic compounds that are formed when one or more sites on a benzene ring, substitute their hydrogen atoms in exchange for the corresponding number of \[ - OH\] or alcoholic functional group.
Sodium borohydride is a weak reducing agent; it cannot reduce the electron-rich phenol to prepare benzene. But phenol can be converted into benzene by reaction with Zinc dust. the reaction is shown below,
\[{C_6}{H_5}OH\,\,\xrightarrow{{Zn\,\,Dust/\Delta }}{C_6}{H_6}\]
So, the given statement is false.
The correct option is B.
Additional information:
Now, phenols are formed as a product in various reactions, but it is not possible to completely extract all of the phenol formed from these reactions.
Hence, certain methods are developed just to maximize the extraction of phenol from a given chemical reaction. One such process is known as the Cumene process.
Cumene process for manufacturing phenol has many names including cumene – phenol process and Hock process. This method is used for producing both phenol and acetone. The reactants used in this reaction are benzene and propylene. Another important reactant required is oxygen, which is obtained from the air. Also, to be included is a small amount of radical initiators. This method is used all over the world for the production of phenol. The chemical reaction involved in this process is as follows:

Note:The cumene process is a widely used method for the production of phenol. It involves synthesizing two relatively cheap compounds, viz. Benzene and propylene, into two extremely useful products like phenol and acetone. The most important chemical made from phenol is bisphenol A, which is used to make the polycarbonates. Phenol is also catalytically reduced to cyclohexanol which is used in the manufacture of polyamides 6 and 6, 6.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




