
The reddish brown liquid ‘S’ is dissolved in water. When ethyne gas is passed through it, ‘S’ turns colourless. Find the substances ‘S’ based on the given information.
A) liq. \[{H_2}O\]
B) liq. \[B{r_2}\]
C) liq. \[{I_2}\]
D) liq. \[C{l_2}\]
Answer
502.5k+ views
Hint: As we know that in organic chemistry, hydrocarbons are an important topic. The hydrocarbons are majorly classified as three groups. There are alkane, alkene and alkyne. The alkane means carbon-carbon single bond. The alkene has a carbon-carbon double bond. The alkyne means carbon-carbon having triple bond in the molecule. The general formula of alkane is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}\]. The general formula of alkene is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\]. The general formula of alkyne is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}\].
Complete answer:
We need to know that the molecular formula of ethyne is \[HC \equiv CH\].
The structural formula of ethyne is
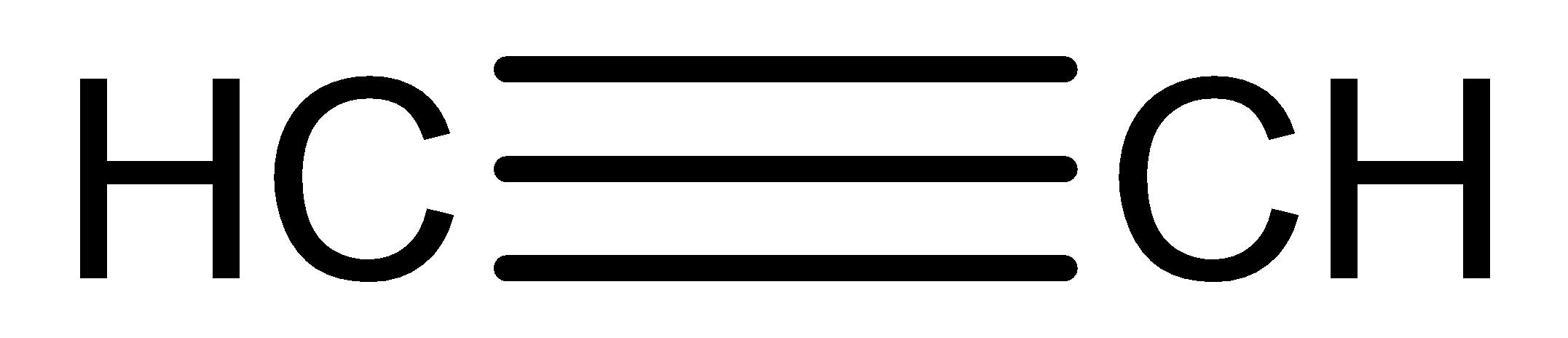
When ethyne is reacted with bromine water to give the product in the colourless form.
The chemical reaction for the above discussion is given below,
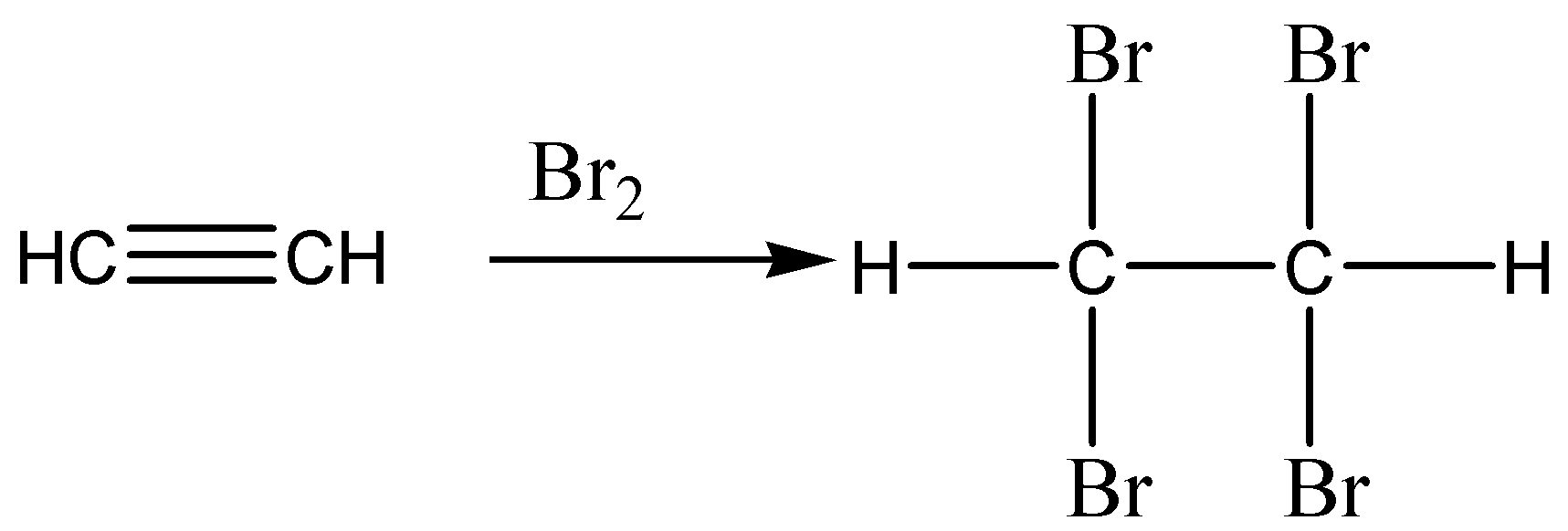
According to the above discussion and chemical reaction we conclude the reddish brown liquid \[B{r_2}\] is dissolved in water and ethyne gas is passed through it, \[B{r_2}\] turns colourless.
Hence, option B is the correct answer.
Note:
We must know the conversion of one type of hydrocarbon to another hydrocarbon by oxidation and reduction. The oxidation of alkane to give alkene. The oxidation of alkene to give alkyne. The reduction of alkyne to give alkene. The reduction of alkene to give alkane. The full form of IUPAC is the International Union of Pure and Applied Chemistry. It is a World Wide organisation for the nomenclature of the new elements in the periodic table and chemical molecules. The IUPAC has certain rules and regulations for the naming of the organic compound. In that method only we named the organic molecules in World level. The naming of any organic molecule depends on the parent saturated hydrocarbon.
Complete answer:
We need to know that the molecular formula of ethyne is \[HC \equiv CH\].
The structural formula of ethyne is
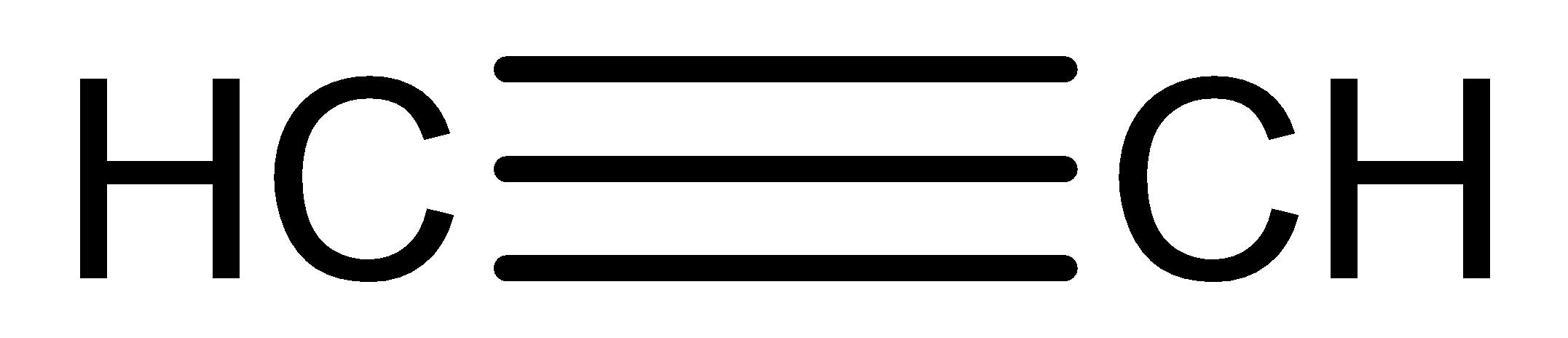
When ethyne is reacted with bromine water to give the product in the colourless form.
The chemical reaction for the above discussion is given below,

According to the above discussion and chemical reaction we conclude the reddish brown liquid \[B{r_2}\] is dissolved in water and ethyne gas is passed through it, \[B{r_2}\] turns colourless.
Hence, option B is the correct answer.
Note:
We must know the conversion of one type of hydrocarbon to another hydrocarbon by oxidation and reduction. The oxidation of alkane to give alkene. The oxidation of alkene to give alkyne. The reduction of alkyne to give alkene. The reduction of alkene to give alkane. The full form of IUPAC is the International Union of Pure and Applied Chemistry. It is a World Wide organisation for the nomenclature of the new elements in the periodic table and chemical molecules. The IUPAC has certain rules and regulations for the naming of the organic compound. In that method only we named the organic molecules in World level. The naming of any organic molecule depends on the parent saturated hydrocarbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




