
The reagent used for converting benzene to ethylbenzene is:
A. \[{C_2}{H_5}CI\] , anhydrous \[AlC{l_3}\]
B. \[{C_2}{H_5}CI\] , aq \[AlC{l_3}\]
C. \[{C_2}{H_5}OH\] , anhydrous \[AlC{l_3}\]
D. \[{C_2}{H_5}CI\] , \[SOC{l_2}\]
Answer
582.3k+ views
Hint: Reagent can be a substance or mixture of compounds which are basically used in chemical analysis or reactions. When a reagent is added to a system it causes chemical reactions. For a particular reaction a particular reagent gets consumed in the process of the chemical reaction.
Complete step by step answer:
There are so many different kinds of reaction sequence pathways to do this conversion. The use of every reagent which is used in conversions should be known. Always find out the change of groups and make a suitable reaction sequence for conversions.
To convert benzene to ethylbenzene there is a very well-known reaction which is the Friedel-Craft reaction. It is basically a coupling reaction between aromatic compounds (benzene) and alkyl halide or acyl halide in presence of anhydrous Lewis acid \[AlC{l_3}\] . In presence of aq. \[AlC{l_3}\] it reduces the nucleophilicity of the benzene ring by formation of cation of benzene ring. The reaction is shown below,
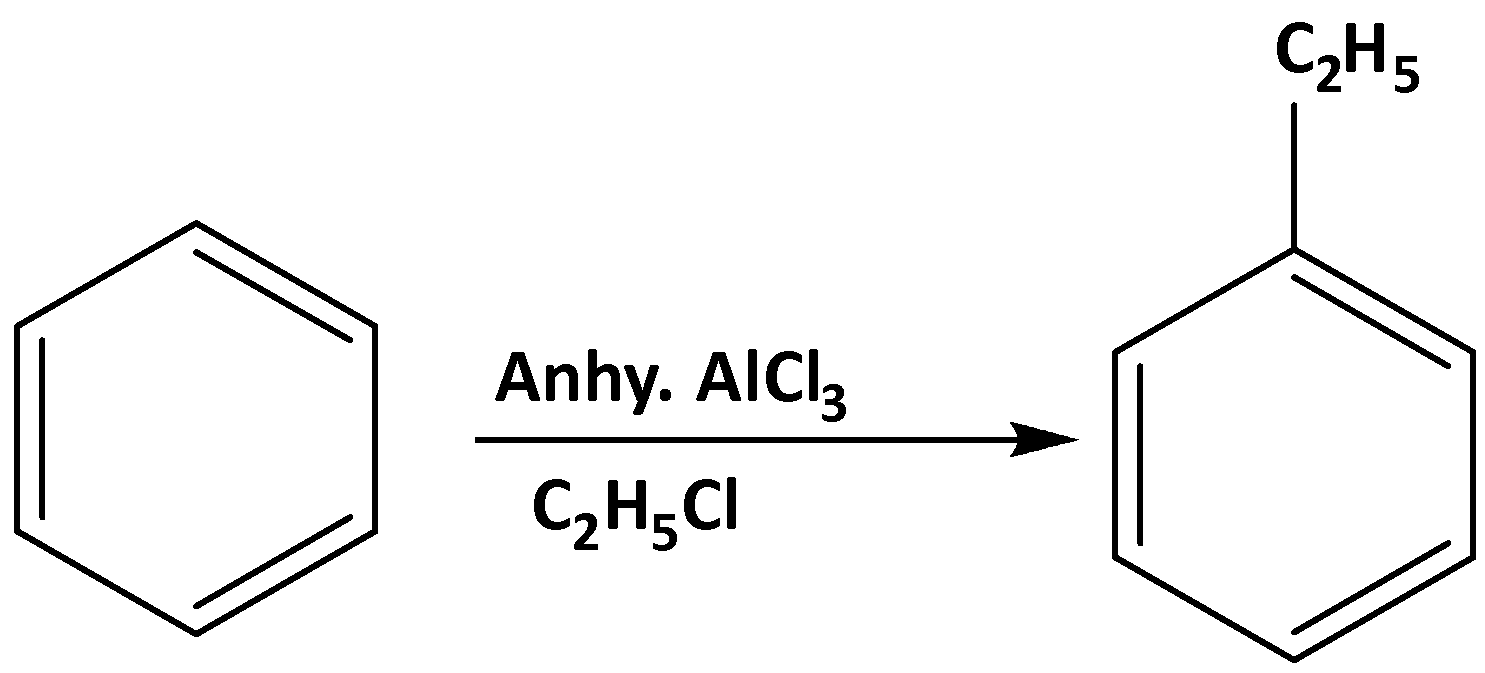
Therefore, the reagent used for converting benzene to ethylbenzene is: \[{C_2}{H_5}CI\] , anhydrous \[AlC{l_3}\]
So, the correct option is A.
Note: Friedel-Craft reaction with aryl halide is not possible because the lone pair of halogen undergoes conjugation with the benzene ring and forms a partial double bond. Due to this reason, only alkyl halide can be used as a halide component in a Friedel-craft reaction.
Complete step by step answer:
There are so many different kinds of reaction sequence pathways to do this conversion. The use of every reagent which is used in conversions should be known. Always find out the change of groups and make a suitable reaction sequence for conversions.
To convert benzene to ethylbenzene there is a very well-known reaction which is the Friedel-Craft reaction. It is basically a coupling reaction between aromatic compounds (benzene) and alkyl halide or acyl halide in presence of anhydrous Lewis acid \[AlC{l_3}\] . In presence of aq. \[AlC{l_3}\] it reduces the nucleophilicity of the benzene ring by formation of cation of benzene ring. The reaction is shown below,

Therefore, the reagent used for converting benzene to ethylbenzene is: \[{C_2}{H_5}CI\] , anhydrous \[AlC{l_3}\]
So, the correct option is A.
Note: Friedel-Craft reaction with aryl halide is not possible because the lone pair of halogen undergoes conjugation with the benzene ring and forms a partial double bond. Due to this reason, only alkyl halide can be used as a halide component in a Friedel-craft reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




