
The reaction of sodium acetate and soda lime gives:
A. butane
B. ethane
C. methane
D. propane
Answer
583.8k+ views
Hint: When Soda lime reacts with substrate, decarboxylation from the substrate happens. The formula of soda lime is \[NaOH + CaO\] . Sodium acetate is the sodium salt of acetic acid, which can be formed by reaction of sodium hydroxide which is a base and acetic acid. They undergo acid base reaction and form salt and water.
Complete step by step answer:
This reaction with soda lime is generally used to prepare alkanes from carboxylic acids. In this reaction the number of carbon atoms from the reactant is get decreased by one unit in terms of carbon dioxide
\[\left( {C{O_2}} \right).\]
The reaction equation of this reaction is,
\[C{H_3}COONa\xrightarrow{{NaOH/CaO}}C{H_4} + C{O_{2\,}}\, \uparrow \]
In this reaction \[CaO\] which is lime. It is a gray-white colored solid that can be produced in huge amounts by heating of \[\;CaC{O_3}\] . On heating \[\;CaC{O_3}\] emits \[C{O_2}\] and forms \[CaO\]. This \[CaO\] can easily absorb carbon dioxide at room temperature from the air.
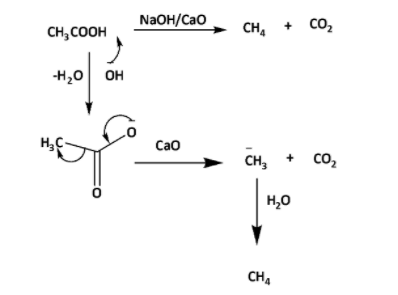
The reaction of sodium acetate with lime gives methane and sodium carbonate.
Hence, option C is correct.
Note:
In the case of \[{{\alpha }}\] -keto carboxylic acid decarboxylation can also be done by normal heating. This is also called oxidative decarboxylation. By this decarboxylation corresponding ketone is formed. The mechanism is shown below.
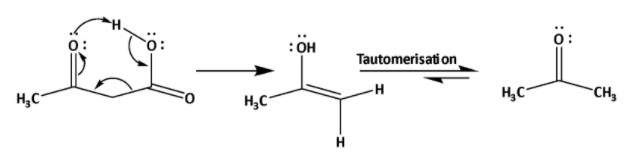
Due to presence of ketone group at alpha position reaction gets fascinated by formation of 6-membered cyclic arrangement for decarboxylation. In this reaction an enol is formed which undergoes tautomerization to form ketone.
Complete step by step answer:
This reaction with soda lime is generally used to prepare alkanes from carboxylic acids. In this reaction the number of carbon atoms from the reactant is get decreased by one unit in terms of carbon dioxide
\[\left( {C{O_2}} \right).\]
The reaction equation of this reaction is,
\[C{H_3}COONa\xrightarrow{{NaOH/CaO}}C{H_4} + C{O_{2\,}}\, \uparrow \]
In this reaction \[CaO\] which is lime. It is a gray-white colored solid that can be produced in huge amounts by heating of \[\;CaC{O_3}\] . On heating \[\;CaC{O_3}\] emits \[C{O_2}\] and forms \[CaO\]. This \[CaO\] can easily absorb carbon dioxide at room temperature from the air.

The reaction of sodium acetate with lime gives methane and sodium carbonate.
Hence, option C is correct.
Note:
In the case of \[{{\alpha }}\] -keto carboxylic acid decarboxylation can also be done by normal heating. This is also called oxidative decarboxylation. By this decarboxylation corresponding ketone is formed. The mechanism is shown below.

Due to presence of ketone group at alpha position reaction gets fascinated by formation of 6-membered cyclic arrangement for decarboxylation. In this reaction an enol is formed which undergoes tautomerization to form ketone.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




