
The reaction of $\text{ }{{\text{P}}_{\text{4}}}\text{ }$with X leads selectively to$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ }$. The X is:
A) Dry $\text{ }{{\text{O}}_{\text{2}}}\text{ }$
B) A mixture of $\text{ }{{\text{O}}_{\text{2}}}\text{ }$ and $\text{ }{{\text{N}}_{\text{2}}}\text{ }$
C) Moist $\text{ }{{\text{O}}_{\text{2}}}\text{ }$
D) $\text{ }{{\text{O}}_{\text{2}}}\text{ }$ in the presence of aqueous $\text{ NaOH }$
Answer
581.4k+ views
Hint: Oxides are the binary compounds of the oxygen with the element. The phosphorus $\text{ }{{\text{P}}_{\text{4}}}\text{ }$ reacts with the air. The phosphorus forms common oxides such as phosphorus trioxide $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$and phosphorus pentoxide$\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{10}}\text{ ) }$.
Complete answer:
Phosphorus exhibits two oxidation states$\text{ }+3\text{ }$ and$\text{ +5 }$.
Phosphorus forms two common oxides namely:
i) Phosphorous trioxide $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$
ii) Phosphorus pentoxide $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{10}}\text{ ) }$
Phosphorus (III) oxide is prepared from phosphorus. The phosphorus trioxide is formed when phosphorus is burnt in a limited supply of air. The preparation reaction is as follows,
\[\text{ }\begin{matrix}
{{\text{P}}_{\text{4}}} & \text{+} & \text{3}{{\text{O}}_{\text{2}}}\text{ (limited)} & \xrightarrow{{{\text{O}}_{\text{2}}}\text{+}{{\text{N}}_{\text{2}}}} & {{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}} \\
(\text{Phosphorus)} & {} & (\text{Air}) & {} & (\text{Phosphorus trioxide)} \\
\end{matrix}\text{ }\]
The phosphorus is heated in the presence of a limited supply of oxygen and nitrogen. The nitrogen gas prevents further oxidation of $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$to the$\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{10}}\text{ ) }$.
Hence, X is a mixture of oxygen $\text{ }{{\text{O}}_{\text{2}}}\text{ }$ and$\text{ }{{\text{N}}_{\text{2}}}\text{ }$.
Hence, (B) is the correct option.
Additional information:
The properties of phosphorus trioxide $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$are as follows:
i) Phosphorus (III) oxide is a crystalline solid with a garlic odour.
ii) It is soluble in carbon disulphide garlic odour.
ii) It is soluble in carbon disulphide, ether, and chloroform.
iii) Heating in the air:
On heating in air, it forms phosphorus pentoxide.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 2}{{\text{O}}_{\text{2}}}\text{ }\to \text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\text{ }$
iv) Action of water:
It dissolves in cold water to give phosphorous acid.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 6}{{\text{H}}_{\text{2}}}\text{O (Cold) }\to \text{ 4}{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{3}}}\text{ }$
It is therefore considered as the anhydride of phosphorus acid. With hot water, it gives phosphoric acid and inflammable phosphine.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 6}{{\text{H}}_{\text{2}}}\text{O (hot)}\to \text{ 3}{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{4}}\text{ + P}{{\text{H}}_{\text{3}}}\text{ }$
v) Action with chlorine:
v) Action with chlorine:
It reacts vigorously with chlorine to form a mixture of phosphoryl chloride and meta phosphoryl chloride.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 4C}{{\text{l}}_{\text{2}}}\text{ }\to \text{ 2POC}{{\text{l}}_{\text{3}}}\text{ + 2P}{{\text{O}}_{\text{2}}}\text{Cl }$
Structure:
Each atom of phosphorus $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$is present at the centre of a tetrahedron (just \[{{\text{P}}_{\text{4}}}\] units in elemental phosphorus ).Each phosphorus atom is covalently bonded to three oxygen atom and each oxygen atom is bonded to two phosphorus atoms. This is as shown in the figure below. It is clear from the structure that the six oxygen atoms lie along the edges of the tetrahedron of P atoms.
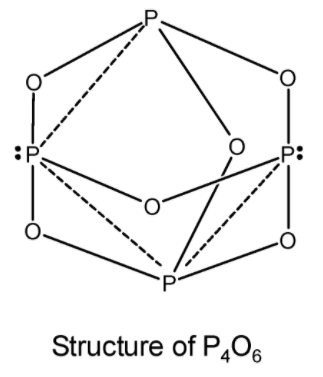
Note: Note that, an excess amount of air $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$is converted into the phosphorus pentoxide$\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$. Apart from trioxide and pentaoxide phosphors have a few more oxide such as $\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{7}}}\text{ }$ ,$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{8\text{ }}}\text{ }$,$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{9 }}}$. The preparation of trioxide is advised to carry out at low temperatures. At high temperature (above $\text{ 21}{{\text{0}}^{\text{0}}}\text{C }$ ) is decomposed into red phosphorus and various other oxides.
Complete answer:
Phosphorus exhibits two oxidation states$\text{ }+3\text{ }$ and$\text{ +5 }$.
Phosphorus forms two common oxides namely:
i) Phosphorous trioxide $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$
ii) Phosphorus pentoxide $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{10}}\text{ ) }$
Phosphorus (III) oxide is prepared from phosphorus. The phosphorus trioxide is formed when phosphorus is burnt in a limited supply of air. The preparation reaction is as follows,
\[\text{ }\begin{matrix}
{{\text{P}}_{\text{4}}} & \text{+} & \text{3}{{\text{O}}_{\text{2}}}\text{ (limited)} & \xrightarrow{{{\text{O}}_{\text{2}}}\text{+}{{\text{N}}_{\text{2}}}} & {{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}} \\
(\text{Phosphorus)} & {} & (\text{Air}) & {} & (\text{Phosphorus trioxide)} \\
\end{matrix}\text{ }\]
The phosphorus is heated in the presence of a limited supply of oxygen and nitrogen. The nitrogen gas prevents further oxidation of $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$to the$\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{10}}\text{ ) }$.
Hence, X is a mixture of oxygen $\text{ }{{\text{O}}_{\text{2}}}\text{ }$ and$\text{ }{{\text{N}}_{\text{2}}}\text{ }$.
Hence, (B) is the correct option.
Additional information:
The properties of phosphorus trioxide $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$are as follows:
i) Phosphorus (III) oxide is a crystalline solid with a garlic odour.
ii) It is soluble in carbon disulphide garlic odour.
ii) It is soluble in carbon disulphide, ether, and chloroform.
iii) Heating in the air:
On heating in air, it forms phosphorus pentoxide.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 2}{{\text{O}}_{\text{2}}}\text{ }\to \text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{10}}}\text{ }$
iv) Action of water:
It dissolves in cold water to give phosphorous acid.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 6}{{\text{H}}_{\text{2}}}\text{O (Cold) }\to \text{ 4}{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{3}}}\text{ }$
It is therefore considered as the anhydride of phosphorus acid. With hot water, it gives phosphoric acid and inflammable phosphine.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 6}{{\text{H}}_{\text{2}}}\text{O (hot)}\to \text{ 3}{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{4}}\text{ + P}{{\text{H}}_{\text{3}}}\text{ }$
v) Action with chlorine:
v) Action with chlorine:
It reacts vigorously with chlorine to form a mixture of phosphoryl chloride and meta phosphoryl chloride.
$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ + 4C}{{\text{l}}_{\text{2}}}\text{ }\to \text{ 2POC}{{\text{l}}_{\text{3}}}\text{ + 2P}{{\text{O}}_{\text{2}}}\text{Cl }$
Structure:
Each atom of phosphorus $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$is present at the centre of a tetrahedron (just \[{{\text{P}}_{\text{4}}}\] units in elemental phosphorus ).Each phosphorus atom is covalently bonded to three oxygen atom and each oxygen atom is bonded to two phosphorus atoms. This is as shown in the figure below. It is clear from the structure that the six oxygen atoms lie along the edges of the tetrahedron of P atoms.

Note: Note that, an excess amount of air $\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$is converted into the phosphorus pentoxide$\text{ ( }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{6}}}\text{ ) }$. Apart from trioxide and pentaoxide phosphors have a few more oxide such as $\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{7}}}\text{ }$ ,$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{8\text{ }}}\text{ }$,$\text{ }{{\text{P}}_{\text{4}}}{{\text{O}}_{\text{9 }}}$. The preparation of trioxide is advised to carry out at low temperatures. At high temperature (above $\text{ 21}{{\text{0}}^{\text{0}}}\text{C }$ ) is decomposed into red phosphorus and various other oxides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




