
The reaction of ethyl magnesium bromide with water would give (ethane / methane / propane)?
Answer
590.7k+ views
Hint: Ethyl magnesium bromide is a Grignard Reagent.Grignard reagent has the formula RMgX where X is a halogen, and R is an alkyl or aryl group.
Complete step by step answer:
The structure of ethyl magnesium bromide or Grignard reagent is –
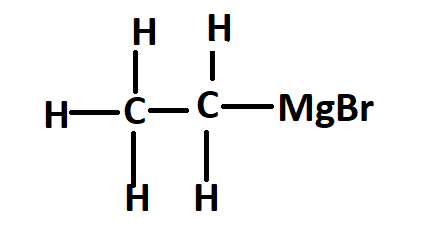
Ethylmagnesium bromide is a Grignard reagent with formula \[C_2H_5MgBr\]. It is widely used in the laboratory synthesis of many organic compounds.
Grignard reagents are made by adding halogenoalkane to magnesium in a flask having ethoxy – ethane (commonly known as diethyl ether or simply "ether"). The flask has a reflux condenser and the mixture is warmed over a water bath for nearly 20 - 30 minutes. Everything has to be perfectly dry because Grignard reagents react with water.
$ \Rightarrow CH_3CH_2Br + Mg\xrightarrow{{ethoxyethane}}CH_3CH_2MgBr$
It is used as a strong base to remove protons from various substrates such as alkynes –
$ \Rightarrow RC \equiv CH + EtMgBr \to RC \equiv CMgBr + EtH$
Ethyl magnesium bromide is a good nucleophile, i.e., it is attracted to electron deficient chemical species.
Grignard reagents react with water to produce alkanes.
$ \Rightarrow CH_3CH_2MgBr + H2O \to CH_3CH_3 + Mg(OH)Br$
The reaction of ethyl magnesium bromide with water will produce ethane. This is a wasteful process.
Additional information:
The inorganic product of this reaction, \[Mg\left( {OH} \right)Br\], is known as a "basic bromide". We can consider it as a sort of half-way stage between magnesium bromide and magnesium hydroxide.
Note:
We should always keep in mind that the Grignard reagent has to be stored in completely dry conditions else the reagent will get converted to their corresponding alkanes.
Complete step by step answer:
The structure of ethyl magnesium bromide or Grignard reagent is –
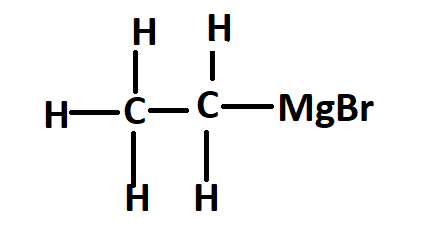
Ethylmagnesium bromide is a Grignard reagent with formula \[C_2H_5MgBr\]. It is widely used in the laboratory synthesis of many organic compounds.
Grignard reagents are made by adding halogenoalkane to magnesium in a flask having ethoxy – ethane (commonly known as diethyl ether or simply "ether"). The flask has a reflux condenser and the mixture is warmed over a water bath for nearly 20 - 30 minutes. Everything has to be perfectly dry because Grignard reagents react with water.
$ \Rightarrow CH_3CH_2Br + Mg\xrightarrow{{ethoxyethane}}CH_3CH_2MgBr$
It is used as a strong base to remove protons from various substrates such as alkynes –
$ \Rightarrow RC \equiv CH + EtMgBr \to RC \equiv CMgBr + EtH$
Ethyl magnesium bromide is a good nucleophile, i.e., it is attracted to electron deficient chemical species.
Grignard reagents react with water to produce alkanes.
$ \Rightarrow CH_3CH_2MgBr + H2O \to CH_3CH_3 + Mg(OH)Br$
The reaction of ethyl magnesium bromide with water will produce ethane. This is a wasteful process.
Additional information:
The inorganic product of this reaction, \[Mg\left( {OH} \right)Br\], is known as a "basic bromide". We can consider it as a sort of half-way stage between magnesium bromide and magnesium hydroxide.
Note:
We should always keep in mind that the Grignard reagent has to be stored in completely dry conditions else the reagent will get converted to their corresponding alkanes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




