
The proportion of sigma, and pi bond in 1,4 – benzoquinone?
A. \[1:3\]
B. \[3:2\]
C. \[2:1\]
D. \[2:3\]
Answer
579k+ views
Hint: To solve this question, we need to first understand the meaning of both sigma and pi bonds. Then, we need to draw the molecular structure of 1,4 – benzoquinone to identify the number of sigma and pi bonds.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Covalent bonds are formed when there is a sharing of electrons between two combining atoms. This sharing of electrons improves the stability of both the combining atoms. Both sigma bonds and pi bonds are formed in covalent compounds. Let us discuss them one by one:
1.Sigma bonds: Sigma bonds are formed by the direct overlapping atomic orbitals. This overlapping happens on the axial planes of both the combining atomic orbitals. Such a heads on overlapping takes place between s and s orbitals, and p and s orbitals. Sigma bonds are the strongest form of covalent bonds. These bonds are also commonly referred to as single bonds.
2.Pi bonds: Pi bonds are formed due to sidewise overlap of atomic orbitals. This bonding takes place over a direction which is perpendicular to the internuclear axis. When pi bonds are being formed, the axes of the atomic orbitals are parallel to each other. Pi bonds are weaker than sigma bonds. Pi bonds are also commonly referred to as double or triple bonds.
The molecular structure of 1,4 – benzoquinone can be given as:
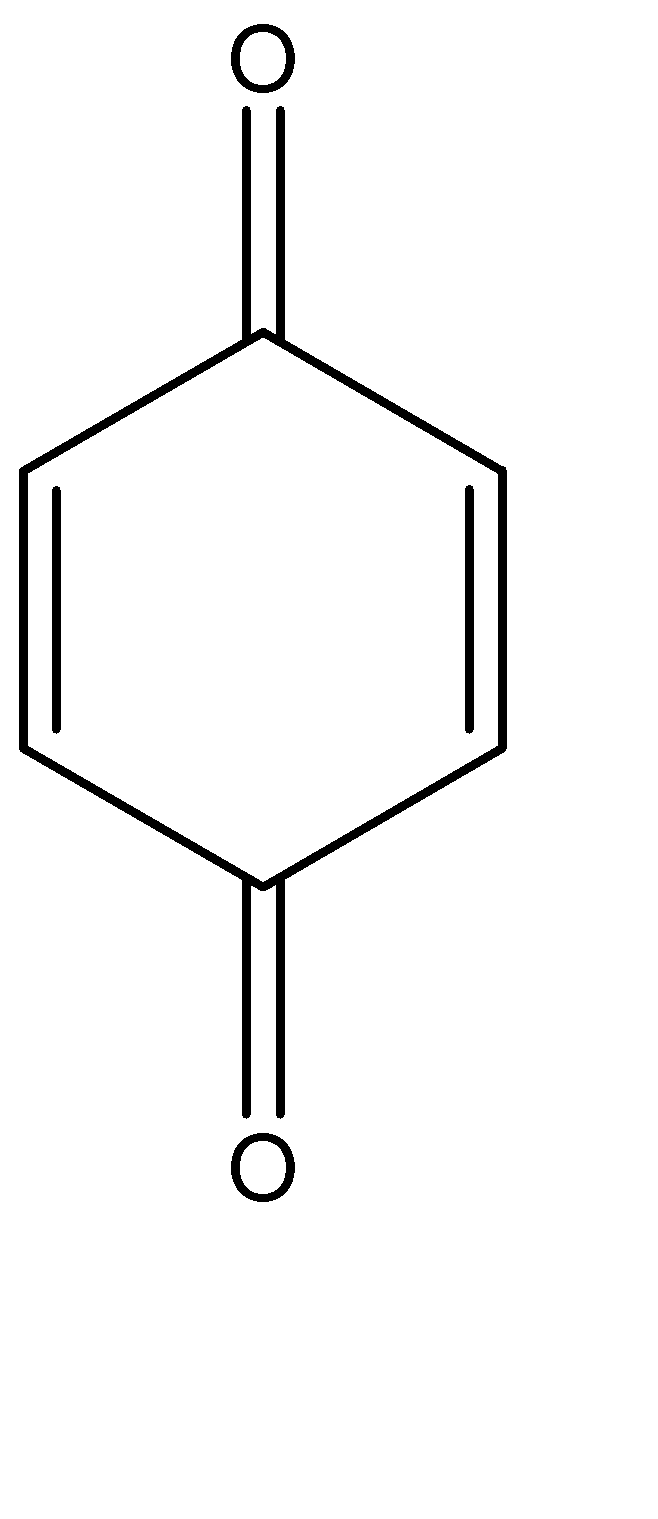
We can see that there are:
a)6 carbon – carbon single bonds
b)2 carbon – oxygen single bonds
c)2 carbon – carbon double bonds
d)2 carbon – oxygen double bonds
Hence, there are 8 single bonds and 4 double bonds present, there are 8 sigma bonds and 4 pi bonds present and the proportion of sigma, and pi bond in 1,4 – benzoquinone is \[2:1\]
Hence, Option C is the correct option.
Note: Double bonds consist of one sigma and one pi bond, whereas a typical triple bond is made up of two pi bonds and one sigma bond. It is important to note that a combination of sigma and pi bonds is always stronger than a single sigma bond.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Covalent bonds are formed when there is a sharing of electrons between two combining atoms. This sharing of electrons improves the stability of both the combining atoms. Both sigma bonds and pi bonds are formed in covalent compounds. Let us discuss them one by one:
1.Sigma bonds: Sigma bonds are formed by the direct overlapping atomic orbitals. This overlapping happens on the axial planes of both the combining atomic orbitals. Such a heads on overlapping takes place between s and s orbitals, and p and s orbitals. Sigma bonds are the strongest form of covalent bonds. These bonds are also commonly referred to as single bonds.
2.Pi bonds: Pi bonds are formed due to sidewise overlap of atomic orbitals. This bonding takes place over a direction which is perpendicular to the internuclear axis. When pi bonds are being formed, the axes of the atomic orbitals are parallel to each other. Pi bonds are weaker than sigma bonds. Pi bonds are also commonly referred to as double or triple bonds.
The molecular structure of 1,4 – benzoquinone can be given as:

We can see that there are:
a)6 carbon – carbon single bonds
b)2 carbon – oxygen single bonds
c)2 carbon – carbon double bonds
d)2 carbon – oxygen double bonds
Hence, there are 8 single bonds and 4 double bonds present, there are 8 sigma bonds and 4 pi bonds present and the proportion of sigma, and pi bond in 1,4 – benzoquinone is \[2:1\]
Hence, Option C is the correct option.
Note: Double bonds consist of one sigma and one pi bond, whereas a typical triple bond is made up of two pi bonds and one sigma bond. It is important to note that a combination of sigma and pi bonds is always stronger than a single sigma bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




