
The product X on oxidation with $HI{O_4}$ gives
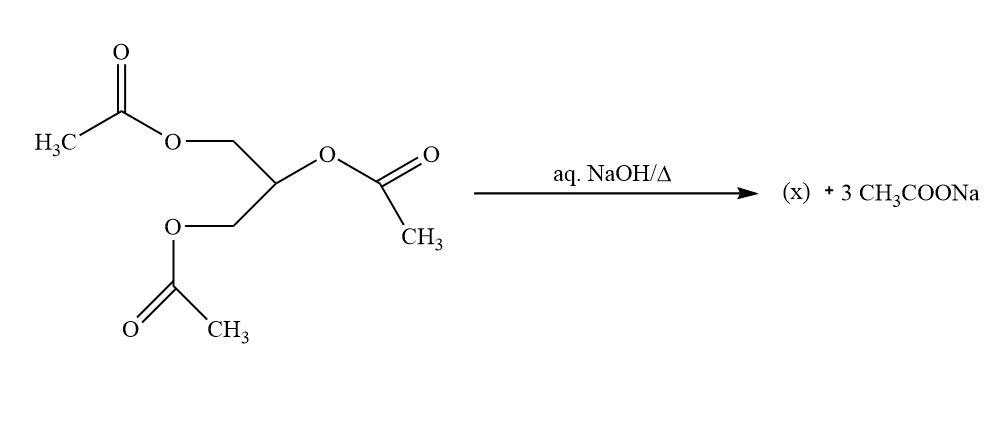
A. $HCHO$
B. $C{H_3}CHO$
C. $C{O_2}$
D. $HCOOH$

Answer
579.6k+ views
Hint: $HI{O_4}$ is known as periodic acid.It is the compound of iodine which has the highest oxides state of seven. It is an oxoacid of iodine. It can exist in two forms: orthoperiodic acid and met aperiodic.
Complete step by step solution
The periodic when reacts with an ester then hydrolysis of the ester group takes place which also takes place in saponification reaction. Soap is being prepared by this saponification process. In this saponification, ester is going to react with base results in the formation of soap along with alcohol.
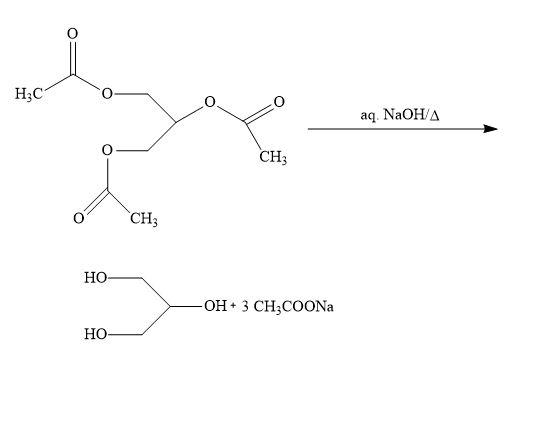
In the above reaction, we have triglyceride that will react with sodium hydroxide (strong base), then glycerol is formed with soap. The name of the soap produced is sodium palmitate.
Now, periodic is a good oxidising agent.it is used to cleave the bond between vicinal diols. When glucose is treated with periodic acid, then there is a formation of formic acid along with formaldehyde. When fructose is treated with periodic acid then there will be a formation of formic acid along with formaldehyde and carbon dioxide. When a molecule contains a terminal hydroxyl group adjacent to another carbon connected to hydroxyl group, then there will be a formation of formaldehyde and an aldol is formed.
And in case of compounds having three consecutive hydroxyl groups then the terminal carbon is oxidised to carbon.
Hence, option (C) is correct.
Note:
The periodic acid helps in the structural analysis of sugars. Whenever there is a presence of vicinal diols then periodic acid is used for oxidation of the molecules. This led to the formation of different complex fragments. After the studying of fragments, we can comment upon the structure.
Complete step by step solution
The periodic when reacts with an ester then hydrolysis of the ester group takes place which also takes place in saponification reaction. Soap is being prepared by this saponification process. In this saponification, ester is going to react with base results in the formation of soap along with alcohol.

In the above reaction, we have triglyceride that will react with sodium hydroxide (strong base), then glycerol is formed with soap. The name of the soap produced is sodium palmitate.
Now, periodic is a good oxidising agent.it is used to cleave the bond between vicinal diols. When glucose is treated with periodic acid, then there is a formation of formic acid along with formaldehyde. When fructose is treated with periodic acid then there will be a formation of formic acid along with formaldehyde and carbon dioxide. When a molecule contains a terminal hydroxyl group adjacent to another carbon connected to hydroxyl group, then there will be a formation of formaldehyde and an aldol is formed.
And in case of compounds having three consecutive hydroxyl groups then the terminal carbon is oxidised to carbon.
Hence, option (C) is correct.
Note:
The periodic acid helps in the structural analysis of sugars. Whenever there is a presence of vicinal diols then periodic acid is used for oxidation of the molecules. This led to the formation of different complex fragments. After the studying of fragments, we can comment upon the structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




