
The product predominates in?
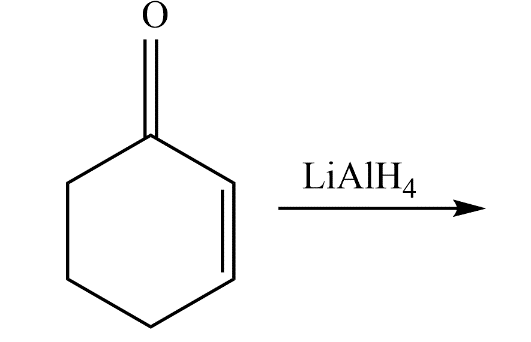
a)
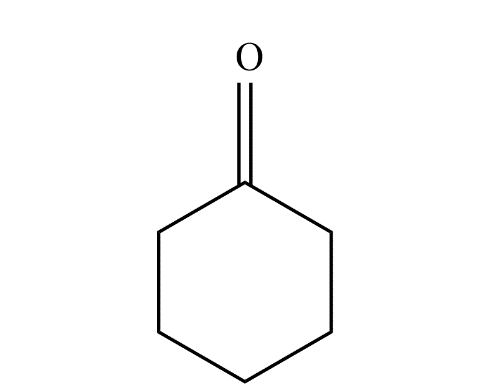
b)
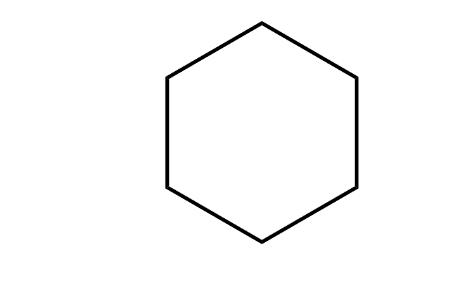
c)
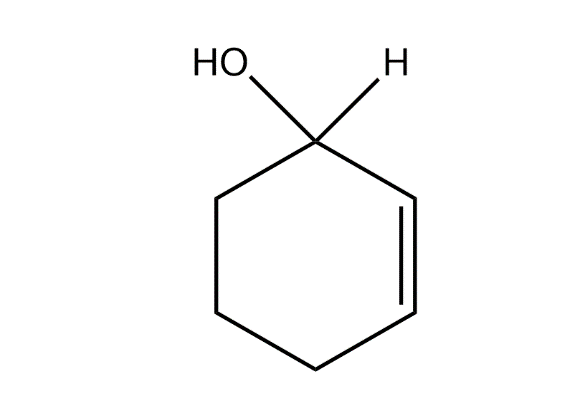
d)





Answer
515.4k+ views
Hint: The given question has a benzene group with a functional group which is a carbonyl group. The reagent used is $ LiAl{H_4}\ $ which is a strong reducing agent. It is also abbreviated as $ LAH\ $ in modern organic synthesis.
Complete answer:
In this reaction, the $ C = O $ gets reduced in presence of $ LiAl{H_4}\ $ and the bond breaks. The electron of that bond moves to oxygen where an attack of hydrogen occurs. This way the Oh bond is established. The other hydrogen forms a bond with the carbocation and hence all the electrons are fully filled. we also observe that there is another double bond present, actually this reducing agent can not reduce an isolated non-polar multiple bond like $ c = c $ . Therefore, the other bond will not reduce.
$ {C_6}{H_5} - CH = CH - CHO\xrightarrow{{LiAl{H_4}}}{C_6}{H_5}C{H_2}C{H_2}C{H_2}OH\ $
Reduction of alpha and beta unsaturated carbonyl compounds generally gives 1,2-reduction product (i.e; double bond or triple bond is not reduced) when a phenyl group is attached to the beta carbon atom of a, b unsaturated carbonyl group, the double bond is also reduced.
$ {C_6}{H_5} - CH = CH - CHO\xrightarrow{{LiAl{H_4}}}{C_6}{H_5}C{H_2}C{H_2}C{H_2}OH\ $
In such cases reduction of the double bond of allylic alcohol is thought to proceed through a cyclic organoaluminum compound. The cyclic intermediate reacts with allyl alcohol to give the product.
$ 2{C_6}{H_5} - CH = CH - CHO\xrightarrow{{LiAl{H_4}}}{({C_6}{H_5}C{H_2}C{H_2}C{H_2}O)_2}Al{H_2}\ $
So option, C is correct.
Note:
$ LiAl{H_4}\ $ is a nucleophilic reducing agent, best used to reduce polar multiple bonds like present in the carbonyl group. This group reduces the aldehydic group into primary alcohol, amides and nitriles into amines, carboxylic acids and esters to primary alcohols, and ketones into secondary alcohols.
Complete answer:
In this reaction, the $ C = O $ gets reduced in presence of $ LiAl{H_4}\ $ and the bond breaks. The electron of that bond moves to oxygen where an attack of hydrogen occurs. This way the Oh bond is established. The other hydrogen forms a bond with the carbocation and hence all the electrons are fully filled. we also observe that there is another double bond present, actually this reducing agent can not reduce an isolated non-polar multiple bond like $ c = c $ . Therefore, the other bond will not reduce.
$ {C_6}{H_5} - CH = CH - CHO\xrightarrow{{LiAl{H_4}}}{C_6}{H_5}C{H_2}C{H_2}C{H_2}OH\ $
Reduction of alpha and beta unsaturated carbonyl compounds generally gives 1,2-reduction product (i.e; double bond or triple bond is not reduced) when a phenyl group is attached to the beta carbon atom of a, b unsaturated carbonyl group, the double bond is also reduced.
$ {C_6}{H_5} - CH = CH - CHO\xrightarrow{{LiAl{H_4}}}{C_6}{H_5}C{H_2}C{H_2}C{H_2}OH\ $
In such cases reduction of the double bond of allylic alcohol is thought to proceed through a cyclic organoaluminum compound. The cyclic intermediate reacts with allyl alcohol to give the product.
$ 2{C_6}{H_5} - CH = CH - CHO\xrightarrow{{LiAl{H_4}}}{({C_6}{H_5}C{H_2}C{H_2}C{H_2}O)_2}Al{H_2}\ $
So option, C is correct.
Note:
$ LiAl{H_4}\ $ is a nucleophilic reducing agent, best used to reduce polar multiple bonds like present in the carbonyl group. This group reduces the aldehydic group into primary alcohol, amides and nitriles into amines, carboxylic acids and esters to primary alcohols, and ketones into secondary alcohols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




