
What will be the product of the following reaction?

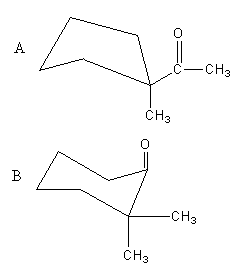
C. Both A and B
D, none of the above


Answer
562.8k+ views
Hint:To determine the answer we should identify the name of the given reaction by looking at its reactant and reagents. Here, the reactant is diol and the reagent is an acid or acidic condition so, the name of this reaction is a pinacol-pinacolone rearrangement. The rearrangement in presence of acid gives the ketone.
Complete solution:
The rearrangement of diol in presence of acid to a ketone is known as pinacol-pinacolone rearrangement.
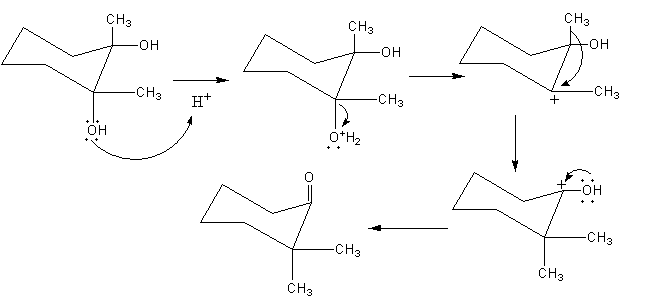
The mechanism of rearrangement is shown as follows:
Initially, the hydroxyl group present at equatorial position is attacked at proton and gets protonated, so a water molecule leaves forming a carbocation. The Trans bond (Trans to the hydroxyl group bond, shown by the red coloured bond) shifts to the carbocation so ring contraction takes place forming a five-membered ring. The carbocation forms at another carbon and hydroxyl group attached with that carbon donating its lone pair to the carbocation. Final removal of a proton from the hydroxyl group forms ketone.

As the reactant has two hydroxyl groups. The hydroxyl group present at the axial position can also attack the proton and get protonated. By the removal of a water molecule, a carbocation will forms. The methyl group at the adjacent carbon is Trans to the carbocation so, methyl group will transfer to the carbocation and the carbocation forms at another carbon and hydroxyl group attached with that carbon donate its lone pair to the carbocation. Final removal of a proton from the hydroxyl group forms ketone.
The mechanism of the rearrangement is shown as follows:
Therefore, the correct answer is (C).
Note:The pinacol-pinacolone rearrangement takes place in acidic medium. The $1,2$ axial-axial position of diol is known as Trans. The Trans $1,2$diol does not give pinacol-pinacolone rearrangement because in Trans $1,2$diol, no migratory group is present. The $1,2$ axial-equatorial position of diol is known as cis. Cis give the pinacole-pinacolone rearrangement. In the absence of a migratory group at Trans position, the ring contraction takes place. The migratory aptitude is H > aryl > alkyl.
Complete solution:
The rearrangement of diol in presence of acid to a ketone is known as pinacol-pinacolone rearrangement.
The mechanism of rearrangement is shown as follows:
Initially, the hydroxyl group present at equatorial position is attacked at proton and gets protonated, so a water molecule leaves forming a carbocation. The Trans bond (Trans to the hydroxyl group bond, shown by the red coloured bond) shifts to the carbocation so ring contraction takes place forming a five-membered ring. The carbocation forms at another carbon and hydroxyl group attached with that carbon donating its lone pair to the carbocation. Final removal of a proton from the hydroxyl group forms ketone.

As the reactant has two hydroxyl groups. The hydroxyl group present at the axial position can also attack the proton and get protonated. By the removal of a water molecule, a carbocation will forms. The methyl group at the adjacent carbon is Trans to the carbocation so, methyl group will transfer to the carbocation and the carbocation forms at another carbon and hydroxyl group attached with that carbon donate its lone pair to the carbocation. Final removal of a proton from the hydroxyl group forms ketone.
The mechanism of the rearrangement is shown as follows:

Therefore, the correct answer is (C).
Note:The pinacol-pinacolone rearrangement takes place in acidic medium. The $1,2$ axial-axial position of diol is known as Trans. The Trans $1,2$diol does not give pinacol-pinacolone rearrangement because in Trans $1,2$diol, no migratory group is present. The $1,2$ axial-equatorial position of diol is known as cis. Cis give the pinacole-pinacolone rearrangement. In the absence of a migratory group at Trans position, the ring contraction takes place. The migratory aptitude is H > aryl > alkyl.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




