
The product of following reaction is
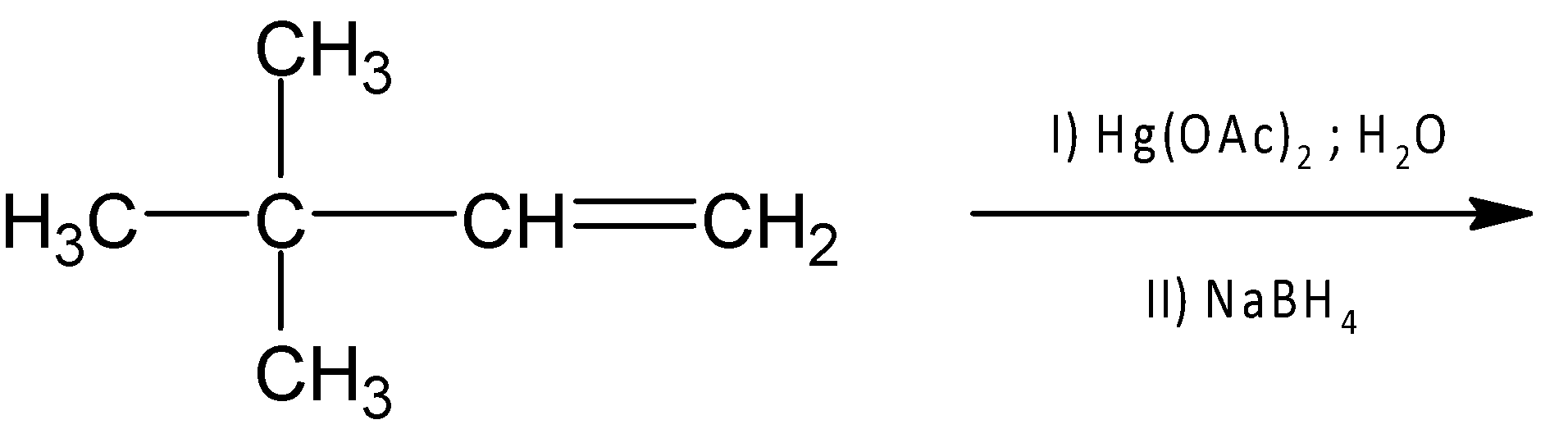
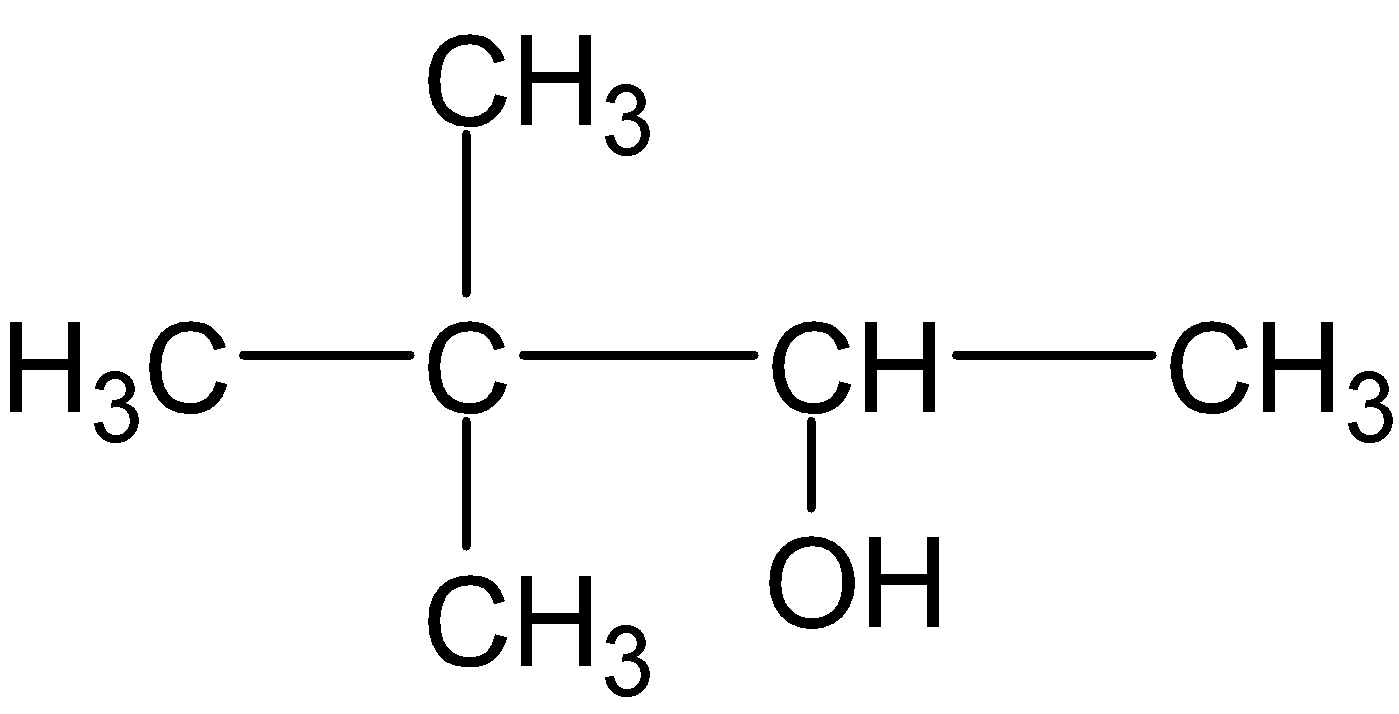
A.
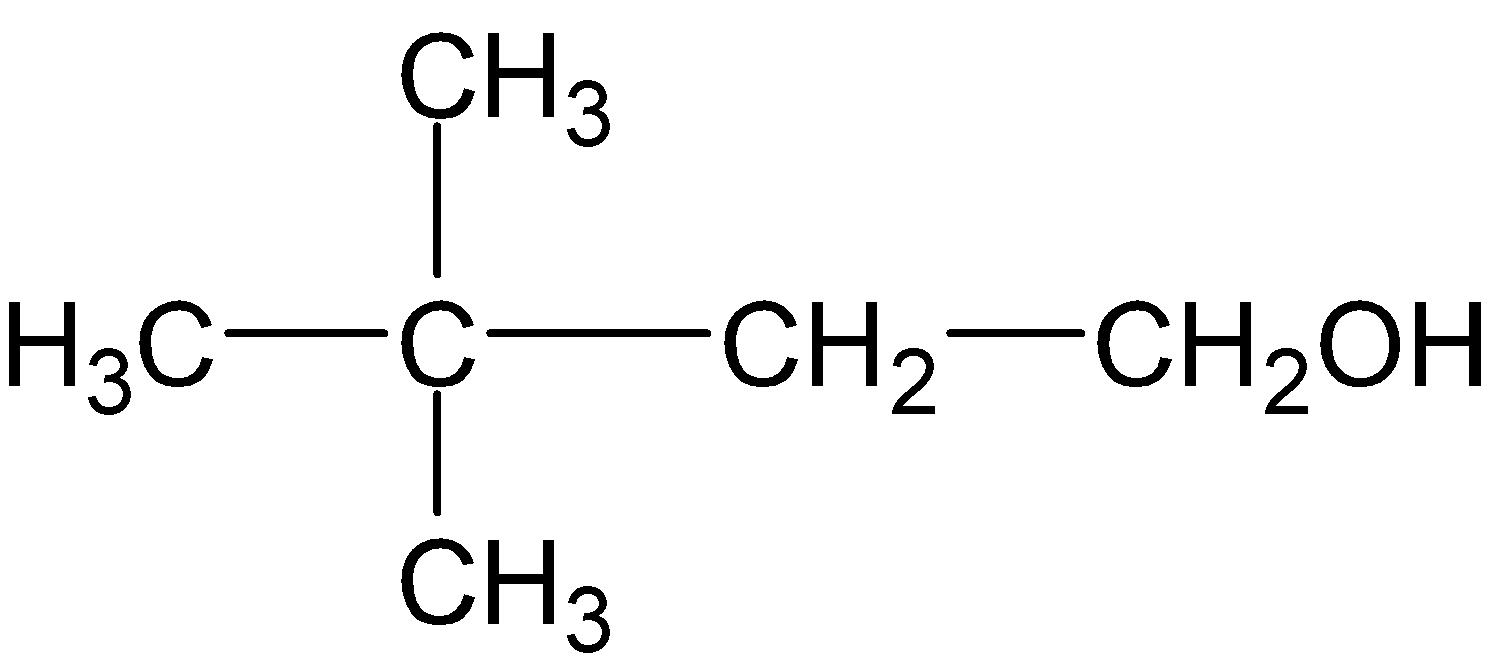
B.
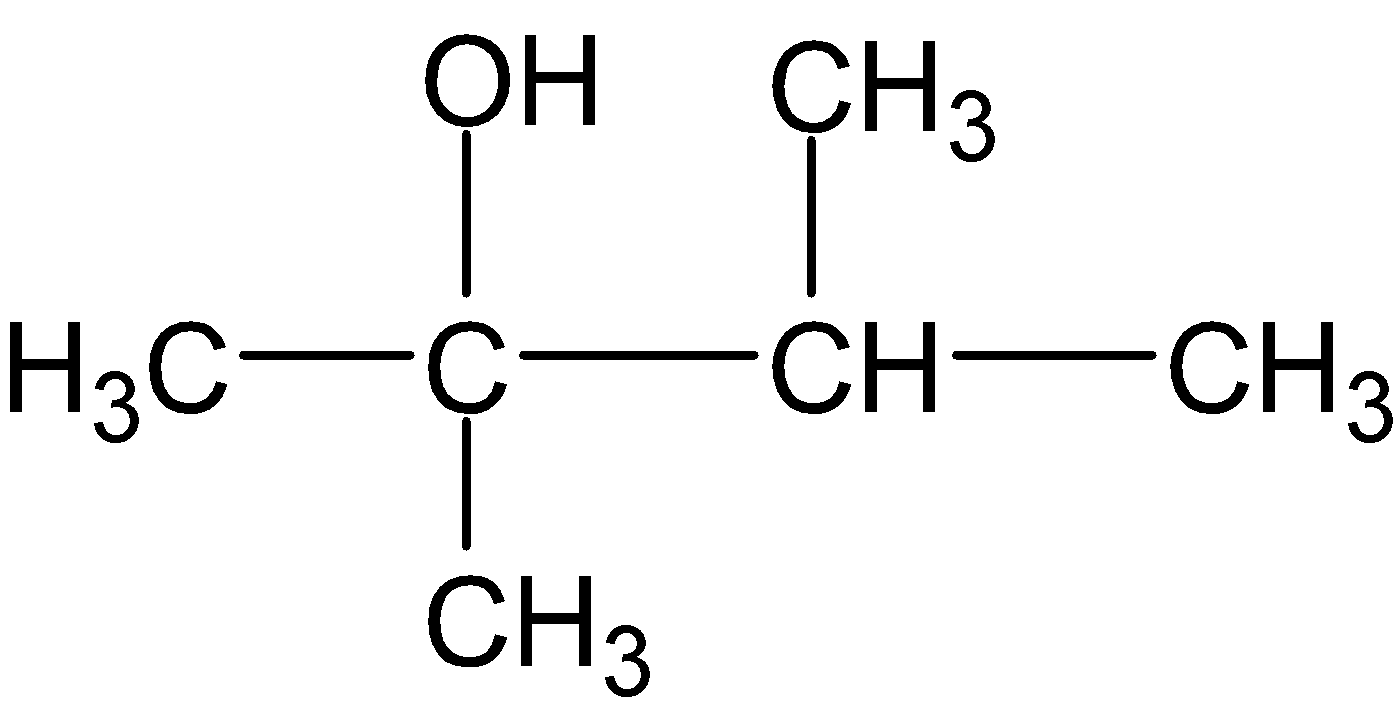
C.
D.

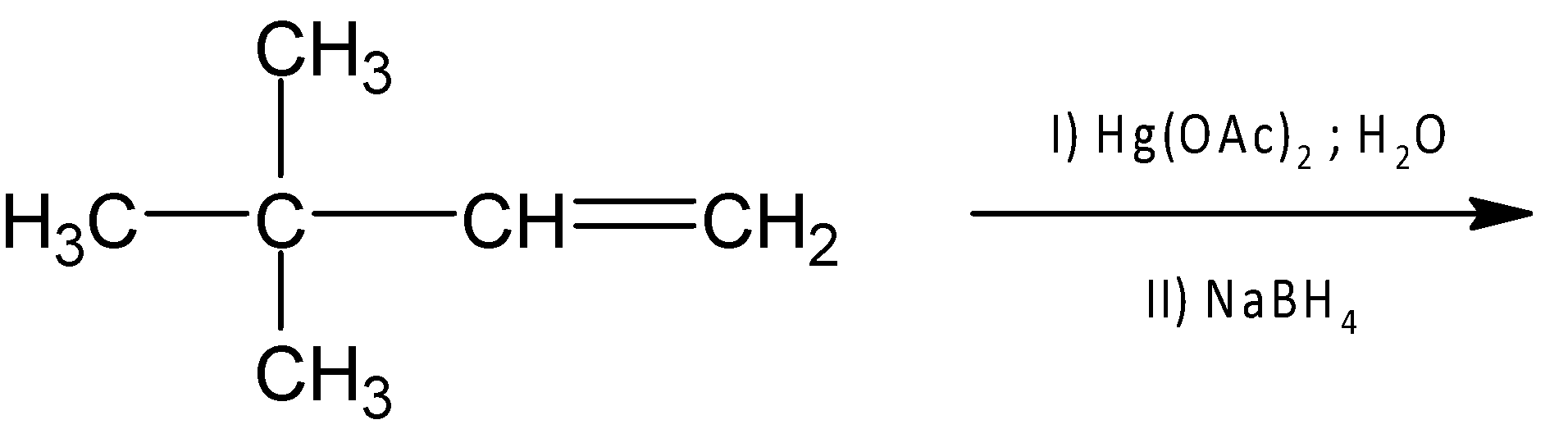




Answer
361.8k+ views
Hint: The process in which alkene to alcohol is done in the presence of $Hg{{(OAc)}_{2}}$ sodium borohydride ($NaB{{H}_{4}}$) is called Oxymercuration-demercuration reaction. The major product is alcohol which is a result of the Markovnikov addition reaction.
Complete Step by Step Answer:
The oxymercuration-demercuration of alkenes provides us with an alternative pathway for the synthesis of Merkovnikov’s alcohol from any alkene. In this process, any alkene compound is treated with mercury(II) acetate $Hg{{(OAc)}_{2}}$and $NaB{{H}_{4}}$ the final product in which hydroxyl group ($-OH$) bonds to the more substituted carbon atom of the alkene compound.
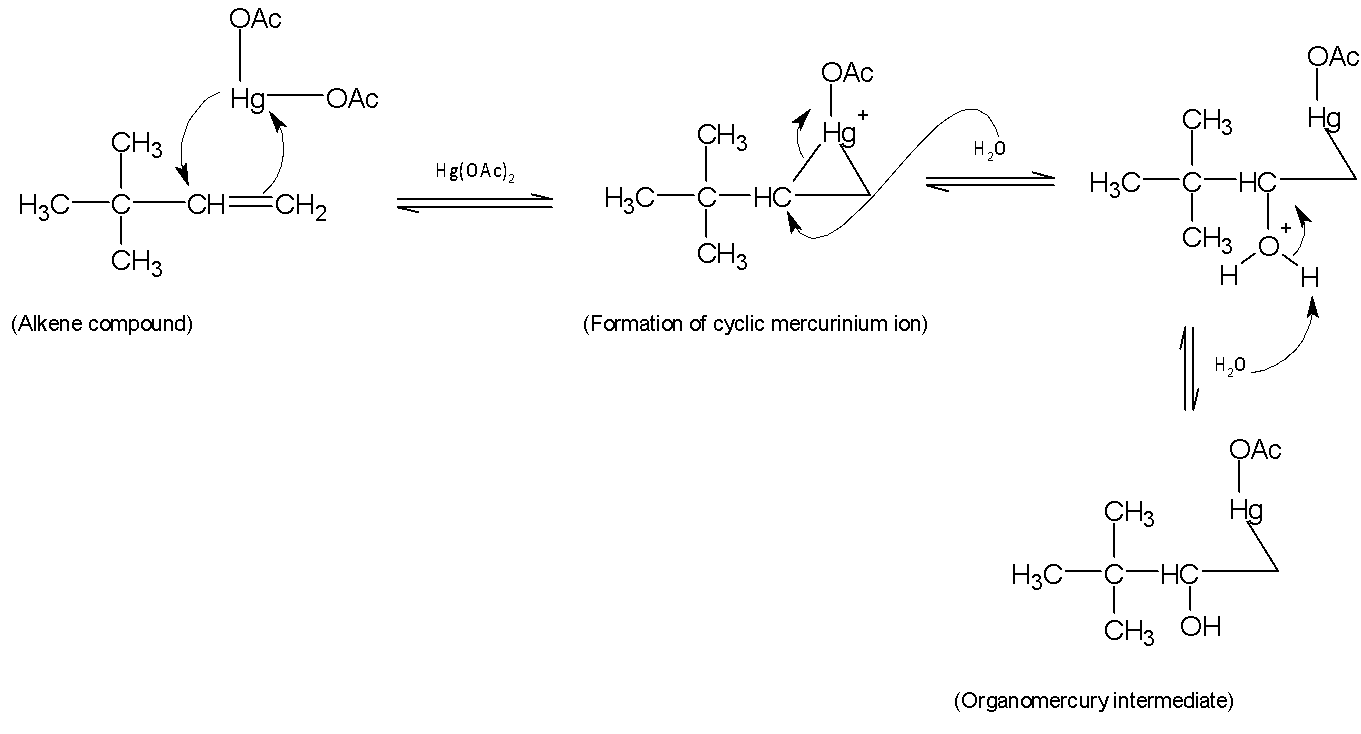
The mechanism of this reaction contains more than one step. In the first step, an electrophile $HgOA{{c}^{+}}$ is generated and gives a cyclic mercurinium ion. A weakly nucleophilic water attacks on the most substituted carbon to open the three-membered cyclic mercurinium ion bridge followed by proton transfer to a solvent water molecule. The reason behind attacking more substituted carbon centres than the primary carbon centre is that the partial positive charge is better accommodated on tertiary carbon than on primary carbon.
Mechanism:
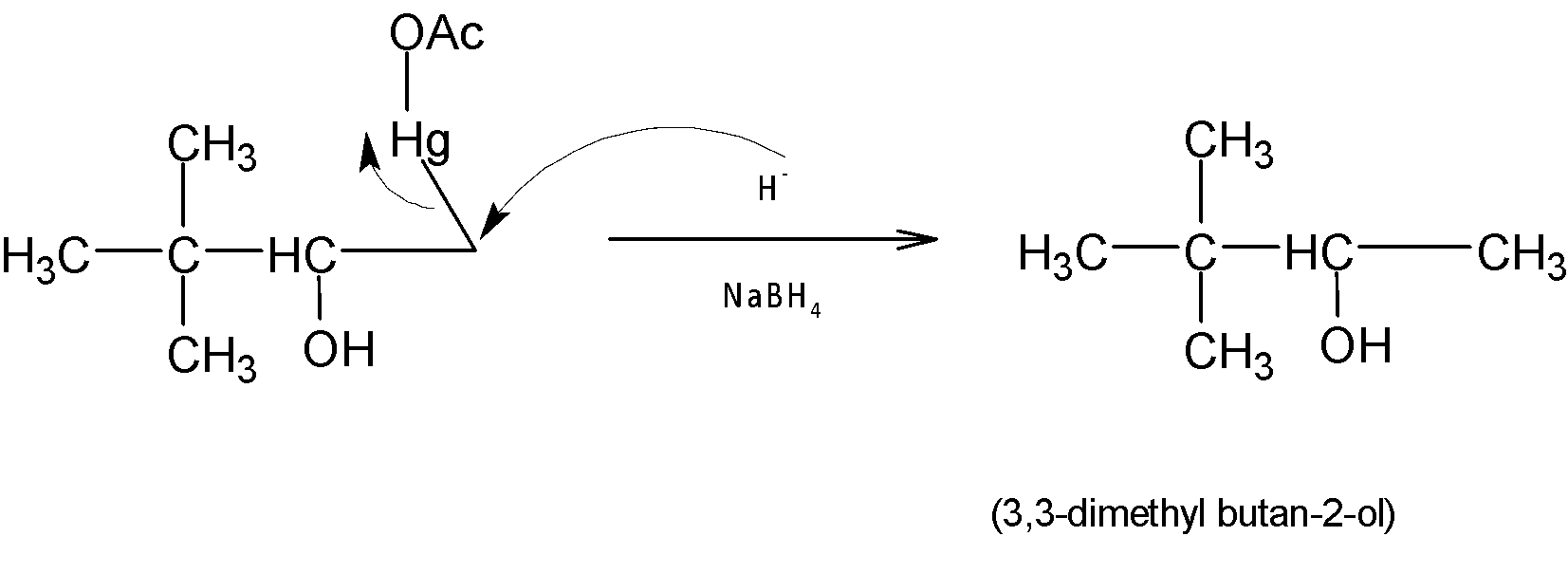
Finally, the organomercury intermediate is further reduced by sodium borohydride $(NaB{{H}_{4}})$ via hydride shift. Hence the major product is $3,3-dimethylbutan-2-ol$.
Therefore , option (A) is correct.
Note: Another process for the reduction of alkene to alcohol is known as hydroboration-oxidation. This is a reduction process of alkene followed by anti-Markovnikov regioselectivity. Here hydroxyl group is attached to the less substituted carbon centre or a carbon centre with less number of hydrogens.
Complete Step by Step Answer:
The oxymercuration-demercuration of alkenes provides us with an alternative pathway for the synthesis of Merkovnikov’s alcohol from any alkene. In this process, any alkene compound is treated with mercury(II) acetate $Hg{{(OAc)}_{2}}$and $NaB{{H}_{4}}$ the final product in which hydroxyl group ($-OH$) bonds to the more substituted carbon atom of the alkene compound.
The mechanism of this reaction contains more than one step. In the first step, an electrophile $HgOA{{c}^{+}}$ is generated and gives a cyclic mercurinium ion. A weakly nucleophilic water attacks on the most substituted carbon to open the three-membered cyclic mercurinium ion bridge followed by proton transfer to a solvent water molecule. The reason behind attacking more substituted carbon centres than the primary carbon centre is that the partial positive charge is better accommodated on tertiary carbon than on primary carbon.
Mechanism:

Finally, the organomercury intermediate is further reduced by sodium borohydride $(NaB{{H}_{4}})$ via hydride shift. Hence the major product is $3,3-dimethylbutan-2-ol$.

Therefore , option (A) is correct.
Note: Another process for the reduction of alkene to alcohol is known as hydroboration-oxidation. This is a reduction process of alkene followed by anti-Markovnikov regioselectivity. Here hydroxyl group is attached to the less substituted carbon centre or a carbon centre with less number of hydrogens.
Recently Updated Pages
Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

The coating formed on the metals such as iron silver class 12 chemistry CBSE

Metals are refined by using different methods Which class 12 chemistry CBSE

What do you understand by denaturation of proteins class 12 chemistry CBSE

Assertion Nitrobenzene is used as a solvent in FriedelCrafts class 12 chemistry CBSE

Trending doubts
What are the factors of 100 class 7 maths CBSE

Which are the Top 10 Largest Countries of the World?

What is BLO What is the full form of BLO class 8 social science CBSE

The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Difference Between Plant Cell and Animal Cell




