
The product is:
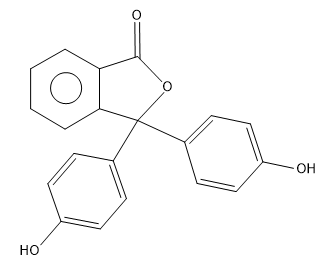
(A)
(B)
(C)
(D) None




Answer
560.7k+ views
Hint: The reaction is a synthesis reaction of an indicator which is commonly used in titrations.
- The reaction is an electrophilic aromatic substitution reaction of phthalic anhydride.
Complete step by step answer:
- From the time we are studying about the organic compounds we have dealt with many compounds, reactions and different functional groups. With the aid of the previous gained basic concepts let's solve the problem.
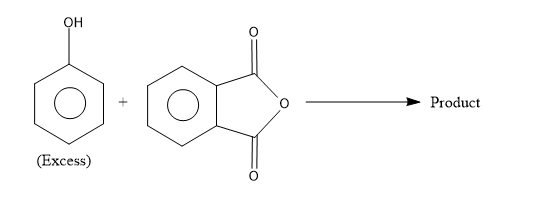
- To complete the chemical reaction, first identify the reactants and the reaction conditions given to predict the product. The reactants given here is a molecule with –OH group attached to the benzene ring which is called as phenol and it is specifically mentioned that phenol is used excess hence we know that more than one molecule of phenol is involved in the reaction. The other reactant is phthalic anhydride.
So first let's see what phthalic anhydride is,as we all know anhydride is an organic compound which is formed by the removal of water from acids.
Here the phthalic anhydride is formed by removing one water molecule from phthalic acid.
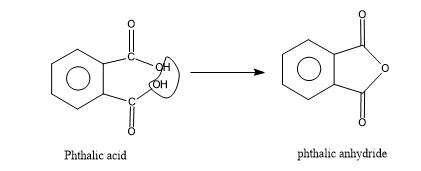
- Now this anhydride is reacting with the excess of phenol to yield an indicator called phenolphthalein.
The phthalic anhydride undergoes the condensation reaction with two molecules of phenol in acidic conditions to yield the phenolphthalein.
The reaction can be written as:
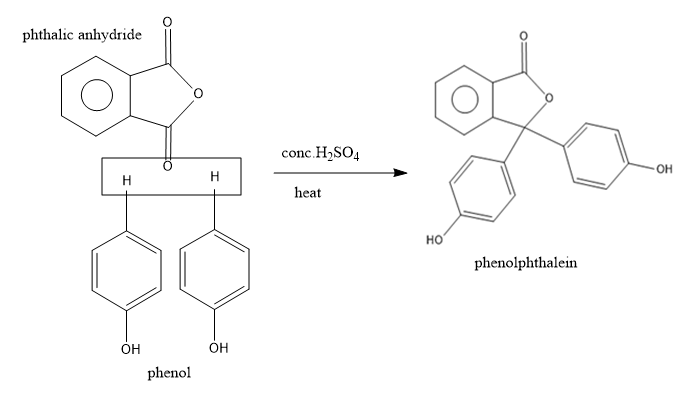
So from the above reaction we can say that the two molecules of phenols combines with one molecule of phthalic anhydride and undergoes condensation reaction and remove one molecule of water by eliminating 2 H atoms from the para position of phenols and one O atom from phthalic anhydride to yield the phenolphthalein.
The correct answer is option “A” .
Note: The phenolphthalein is an indicator used in the acid base reaction.In acidic medium it will be colorless and in basic conditions it is pink in colour.
- Phenolphthalein has different structures in different medium, since the phenolic group is acidic medium, the structure will be as similar as above given but in basic medium we have to remove protons from the structure and resonance stabilized and finally breaks the bonds of phthalide anhydride and exist as:
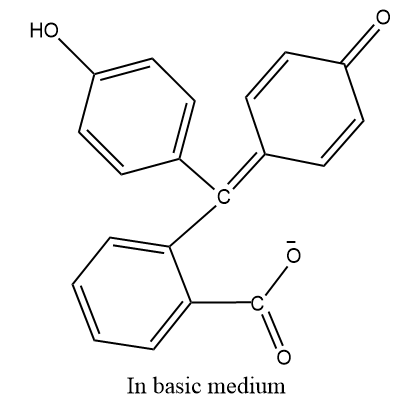
The reaction can be said as the electrophilic substitution reaction as the phthalic anhydride acts as the electrophile here and its substitution takes place at the para position of –OH group of phenol.
- The reaction is an electrophilic aromatic substitution reaction of phthalic anhydride.
Complete step by step answer:
- From the time we are studying about the organic compounds we have dealt with many compounds, reactions and different functional groups. With the aid of the previous gained basic concepts let's solve the problem.
- To complete the chemical reaction, first identify the reactants and the reaction conditions given to predict the product. The reactants given here is a molecule with –OH group attached to the benzene ring which is called as phenol and it is specifically mentioned that phenol is used excess hence we know that more than one molecule of phenol is involved in the reaction. The other reactant is phthalic anhydride.
So first let's see what phthalic anhydride is,as we all know anhydride is an organic compound which is formed by the removal of water from acids.
Here the phthalic anhydride is formed by removing one water molecule from phthalic acid.

- Now this anhydride is reacting with the excess of phenol to yield an indicator called phenolphthalein.
The phthalic anhydride undergoes the condensation reaction with two molecules of phenol in acidic conditions to yield the phenolphthalein.
The reaction can be written as:

So from the above reaction we can say that the two molecules of phenols combines with one molecule of phthalic anhydride and undergoes condensation reaction and remove one molecule of water by eliminating 2 H atoms from the para position of phenols and one O atom from phthalic anhydride to yield the phenolphthalein.
The correct answer is option “A” .
Note: The phenolphthalein is an indicator used in the acid base reaction.In acidic medium it will be colorless and in basic conditions it is pink in colour.
- Phenolphthalein has different structures in different medium, since the phenolic group is acidic medium, the structure will be as similar as above given but in basic medium we have to remove protons from the structure and resonance stabilized and finally breaks the bonds of phthalide anhydride and exist as:

The reaction can be said as the electrophilic substitution reaction as the phthalic anhydride acts as the electrophile here and its substitution takes place at the para position of –OH group of phenol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




