
The product formed by the treatment of ethanol and ethane nitrile in the presence of sulphuric acid is:
(A) Ethyl acetate
(B) Diethyl ether
(C) Ethyl methyl ketone
(D) Butanal
Answer
521.4k+ views
Hint :We know that the sulphuric acid is basically used in chemical reactions as an agent which causes hydrolysis. Usually, hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both substance and water molecule to split into two parts. In such reactions, one fragment of the target molecule (or parent molecule) gains a hydrogen ion.
Complete Step By Step Answer:
Ethanol can be converted to ethanoic acid by the process of oxidation. Oxidation refers to the process of loss of electrons by an atom. For oxidation to occur we use oxidizing agents. The steps followed in the conversion are described as follows: The primary alcohol (ethanol) is oxidized to an aldehyde (ethanal). Then, ethanal is further oxidized and forms carboxylic acid (ethanoic acid).
The nascent oxygen is obtained from the oxidizing agent used during the reaction, which helps in the oxidation. The reaction takes place as depicted below where Ethane nitrile is hydrolyzed to acid which reacts with alcohol to give ester. When the carboxylic acid is added with catalyst and alcohol, an ester is formed along with the water. This is called Fischer esterification.
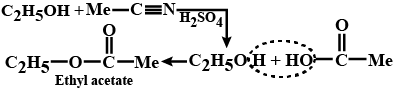
Esters can also be formed by means of esterification reaction. Esters are formed when the carboxylic acid is heated with the alcohol in the presence of a catalyst. In this reaction, the concentrated sulphuric acid is used as a catalyst, a dry form of hydrogen chloride gas is used in some cases.
This method of reaction is used to convert alcohol into an ester. This reaction does not work for the compounds containing the hydroxide Group directly attached to the benzene ring. This method of reaction is called Esterification Reaction.
Therefore, the correct answer is option A.
Note :
Remember that the hydrolysis of nitriles, which are organic molecules containing a cyano group, leads to carboxylic acid formation. These hydrolysis reactions can take place in either acidic or basic solutions. The mechanism for these reactions involves the formation of an amide followed by hydrolysis of the amide to the acid. This is a reversible and slow reaction.
Complete Step By Step Answer:
Ethanol can be converted to ethanoic acid by the process of oxidation. Oxidation refers to the process of loss of electrons by an atom. For oxidation to occur we use oxidizing agents. The steps followed in the conversion are described as follows: The primary alcohol (ethanol) is oxidized to an aldehyde (ethanal). Then, ethanal is further oxidized and forms carboxylic acid (ethanoic acid).
The nascent oxygen is obtained from the oxidizing agent used during the reaction, which helps in the oxidation. The reaction takes place as depicted below where Ethane nitrile is hydrolyzed to acid which reacts with alcohol to give ester. When the carboxylic acid is added with catalyst and alcohol, an ester is formed along with the water. This is called Fischer esterification.

Esters can also be formed by means of esterification reaction. Esters are formed when the carboxylic acid is heated with the alcohol in the presence of a catalyst. In this reaction, the concentrated sulphuric acid is used as a catalyst, a dry form of hydrogen chloride gas is used in some cases.
This method of reaction is used to convert alcohol into an ester. This reaction does not work for the compounds containing the hydroxide Group directly attached to the benzene ring. This method of reaction is called Esterification Reaction.
Therefore, the correct answer is option A.
Note :
Remember that the hydrolysis of nitriles, which are organic molecules containing a cyano group, leads to carboxylic acid formation. These hydrolysis reactions can take place in either acidic or basic solutions. The mechanism for these reactions involves the formation of an amide followed by hydrolysis of the amide to the acid. This is a reversible and slow reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




