
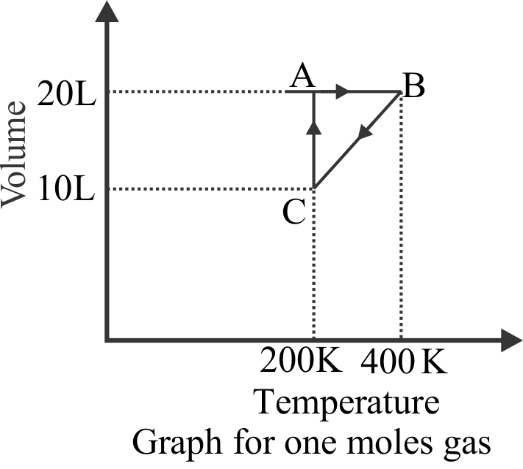
The process which occurs in going from $B \to C$ is
A.Isothermal
B.Adiabatic
C.Isobaric
D.Isochoric

Answer
569.7k+ views
Hint: As we look into the graph of volume versus temperature above we can clearly see that it is a straight line from $C$ to $B$, which says that pressure during the process is constant.
Complete Answer:
Now to be clearer and get the right solution, we have to prove it by using this basic formula,
$PV = nRT$ Or it can be written stating column in the priority
$V = \left( {\dfrac{{nR}}{P}} \right)T$, this is in the form $Y = mx$ where $\because m = \dfrac{{nR}}{P}$
This makes it clear that this is reason of isobaric process
To know it more clearly, let’s find out separately,
${P_B} = \dfrac{{nR{T_B}}}{{{V_B}}} = \dfrac{{nR400}}{{20}} = 20nR$, This is for the $B$, the value is already given in the graph
Now, ${P_C} = \dfrac{{nR{T_C}}}{{{V_C}}} = \dfrac{{nR200}}{{10}} = 20nR$, this is for the $C$, the value is already given in the graph.
Hence, when we see the values, it is the same, so therefore the constant pressure is the same in the process, and this is the process of isobaric.
In thermodynamics, an isobaric process is a type of thermodynamic process in which the pressure of the system stays constant: $\Delta P = 0$. The heat transferred to the system does work, but also changes the internal energy of the system.
This is pretty much the solution. The steps stated the proof and also an alternative method to prove it is an isobaric process. As it was a straight line from $B \to C$, the constant pressure remains the same throughout the whole process which is exactly the definition of isobaric.
So, the correct answer is option C.
Note: in this kind of question setup, one thing to note is that we have to have the knowledge of every small thing and its definition so that during the time it becomes helpful. Graph should be studied well, as the most important hint will always be hidden in graphs or the diagram they give.
Complete Answer:
Now to be clearer and get the right solution, we have to prove it by using this basic formula,
$PV = nRT$ Or it can be written stating column in the priority
$V = \left( {\dfrac{{nR}}{P}} \right)T$, this is in the form $Y = mx$ where $\because m = \dfrac{{nR}}{P}$
This makes it clear that this is reason of isobaric process
To know it more clearly, let’s find out separately,
${P_B} = \dfrac{{nR{T_B}}}{{{V_B}}} = \dfrac{{nR400}}{{20}} = 20nR$, This is for the $B$, the value is already given in the graph
Now, ${P_C} = \dfrac{{nR{T_C}}}{{{V_C}}} = \dfrac{{nR200}}{{10}} = 20nR$, this is for the $C$, the value is already given in the graph.
Hence, when we see the values, it is the same, so therefore the constant pressure is the same in the process, and this is the process of isobaric.
In thermodynamics, an isobaric process is a type of thermodynamic process in which the pressure of the system stays constant: $\Delta P = 0$. The heat transferred to the system does work, but also changes the internal energy of the system.
This is pretty much the solution. The steps stated the proof and also an alternative method to prove it is an isobaric process. As it was a straight line from $B \to C$, the constant pressure remains the same throughout the whole process which is exactly the definition of isobaric.
So, the correct answer is option C.
Note: in this kind of question setup, one thing to note is that we have to have the knowledge of every small thing and its definition so that during the time it becomes helpful. Graph should be studied well, as the most important hint will always be hidden in graphs or the diagram they give.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




