
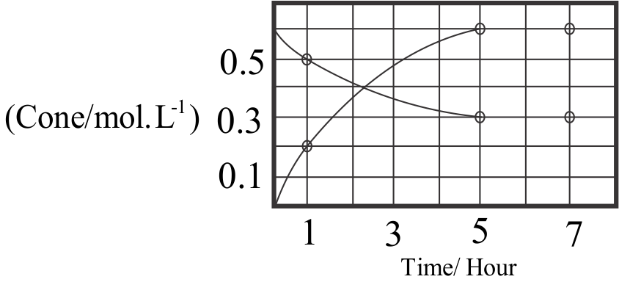
The process of reaction, $A \rightleftharpoons nB$ with time, is represented in the figure, given. Determine the initial rate of conversion of $A$ .

Answer
559.2k+ views
Hint: The initial rate of change for the concentration of $A$ can be given according to the rate of change in concentration with respect to time. The initial rate can be defined for the first part of the reaction process starting from the start of the reaction to only unit time measurements.
Complete step by step answer:
- The rate of change in concentration of $A$ defines the conversion level of $A \rightleftharpoons nB$ . The changing concentration for the first hour is found to be changed to $0.2mol{L^{ - 1}}$ from the initial rate which is $0mol{L^{ - 1}}$ according to the given graphical representation. This change in the concentration takes place over a time of around the first hour.
- This is considered as the initial $1hour$ of the reaction which defines the time for finding out the initiate rate of the reaction. Since this is a reversible reaction there are two molecules involved in the process which are $A$ and $B$ . Therefore, the rate of change takes place for both the molecules in the reaction process.
- The graph depicts the concentration for both the molecules changing with respect to time in hours. The equation for finding out the initial rate of conversion of $A$ is:
For any given reaction process, $Rate = \dfrac{{\Delta Concentration}}{{\Delta Time}}$ .
Here the initial concentration needs to be found out and that is why change is taken for the first $1hour$ as given below, Therefore the resultant initial rate for conversion of $A$ :
$Rate = \dfrac{{0.2 - 0}}{{1 - 0}}$
- Therefore, calculating the value the conversion rate will be $0.2mol{L^{ - 1}}hou{r^{ - 1}}$ . The initial process of conversion may be different from the rates at the final level. This is why only the change for $1hour$ is chosen.
Note: The rate of the reaction can be easily measured based on the concentration to time ratio based on different time intervals. The level of product formation depends on the type of reaction (reversible or non-reversible). In some reactions, the rate is different at different levels but in some of them, the rate remains constant.
Complete step by step answer:
- The rate of change in concentration of $A$ defines the conversion level of $A \rightleftharpoons nB$ . The changing concentration for the first hour is found to be changed to $0.2mol{L^{ - 1}}$ from the initial rate which is $0mol{L^{ - 1}}$ according to the given graphical representation. This change in the concentration takes place over a time of around the first hour.
- This is considered as the initial $1hour$ of the reaction which defines the time for finding out the initiate rate of the reaction. Since this is a reversible reaction there are two molecules involved in the process which are $A$ and $B$ . Therefore, the rate of change takes place for both the molecules in the reaction process.
- The graph depicts the concentration for both the molecules changing with respect to time in hours. The equation for finding out the initial rate of conversion of $A$ is:
For any given reaction process, $Rate = \dfrac{{\Delta Concentration}}{{\Delta Time}}$ .
Here the initial concentration needs to be found out and that is why change is taken for the first $1hour$ as given below, Therefore the resultant initial rate for conversion of $A$ :
$Rate = \dfrac{{0.2 - 0}}{{1 - 0}}$
- Therefore, calculating the value the conversion rate will be $0.2mol{L^{ - 1}}hou{r^{ - 1}}$ . The initial process of conversion may be different from the rates at the final level. This is why only the change for $1hour$ is chosen.
Note: The rate of the reaction can be easily measured based on the concentration to time ratio based on different time intervals. The level of product formation depends on the type of reaction (reversible or non-reversible). In some reactions, the rate is different at different levels but in some of them, the rate remains constant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




