
The process $\Delta U=0$ for an ideal gas can be best represented in the form of a graph:
A.

B.
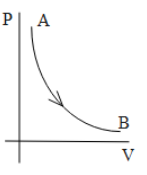
C.

D.




Answer
574.2k+ views
Hint: The study of thermodynamic processes is studied using the laws of thermodynamics. The relation of the change in internal energy of the system is mentioned in the ${{1}^{st}}$ law of thermodynamics. An ideal gas always obeys the law of ideal gas or ideal gas equation.
Formula to be used-
$PV=nRT$
Complete step by step solution:
When the change in the internal energy of a system is zero ($\Delta U=0$ ) the system is said to be an isolated system.
In the case of an isolated system, the temperature is always constant. It is known that the internal energy of a system is directly proportional to the temperature.
$U\propto Temp$
So, if the temperature is content the value of internal energy will be constant.
From the law of ideal gas, we know that,
$PV=nRT$
As in the case of the system mentioned in the question the temperature is constant. So, for this case, the ideal gas equation can be modified as
$PV=constant$
This can be rearranged as
$\begin{align}
& P=\dfrac{contant}{V} \\
& \Rightarrow P\propto \dfrac{1}{V} \\
\end{align}$
As the pressure is inversely proportional to the volume of the system. When the plot of internal pressure will be constructed concerning the volume of the system it will form a hyperbolic curve as shown a=in the graph here.

So, when we give a glance over the graphical representation mentioned in the question.
Hence, the correct option is Option B.
Note:
${{1}^{st}}$ law of thermodynamics is a reconstruction of the law of conservation of energy. According to the law of conservation the energy cannot be created or destroyed it can be transformed from one form to another. In the case of thermodynamic processes, heat is considered to be a form of energy. The thermodynamic equation gives a relation between the internal energy, supplied energy, and the work done by the system ( $\Delta U=Q-W$).
Formula to be used-
$PV=nRT$
Complete step by step solution:
When the change in the internal energy of a system is zero ($\Delta U=0$ ) the system is said to be an isolated system.
In the case of an isolated system, the temperature is always constant. It is known that the internal energy of a system is directly proportional to the temperature.
$U\propto Temp$
So, if the temperature is content the value of internal energy will be constant.
From the law of ideal gas, we know that,
$PV=nRT$
As in the case of the system mentioned in the question the temperature is constant. So, for this case, the ideal gas equation can be modified as
$PV=constant$
This can be rearranged as
$\begin{align}
& P=\dfrac{contant}{V} \\
& \Rightarrow P\propto \dfrac{1}{V} \\
\end{align}$
As the pressure is inversely proportional to the volume of the system. When the plot of internal pressure will be constructed concerning the volume of the system it will form a hyperbolic curve as shown a=in the graph here.

So, when we give a glance over the graphical representation mentioned in the question.
Hence, the correct option is Option B.
Note:
${{1}^{st}}$ law of thermodynamics is a reconstruction of the law of conservation of energy. According to the law of conservation the energy cannot be created or destroyed it can be transformed from one form to another. In the case of thermodynamic processes, heat is considered to be a form of energy. The thermodynamic equation gives a relation between the internal energy, supplied energy, and the work done by the system ( $\Delta U=Q-W$).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




