
The primary prefix is used in order to differentiate a cyclic compound from an acyclic compound.
A.Keto
B.Cyclo
C. Oxo
D. Halo
Answer
584.4k+ views
Hint: IUPAC names are basically combined of primary prefix, root word and primary suffix. By this each compound can be named unique. Primary prefixes are used to indicate the origin of the compound in IUPAC nomenclature. Prefixes are used to differentiate the cyclic and acyclic molecules in IUPAC nomenclature.
Complete step by step answer:
In IUPAC nomenclature, the root word is basically number of total carbons in a longest chain of that compound.
… and so on.
The primary suffix is basically used to differentiate between the saturated compounds (Alkanes) and unsaturated compounds (Alkene and Alkynes).
If there are more than one suffix. Then one of those suffixes is considered a secondary suffix.
Example: Methanol (Alkanol) , here ‘ol’ is a secondary suffix.
The primary prefixes are basically used to differentiate between cyclic compound and noncyclic or chain compound. For cyclic compound prefix s ‘cyclo’. if there are any side chains or groups are present then secondary prefixes like ‘methyl’, ‘ethyl’ are used.
So, the primary prefix ’cyclo’ is used in order to differentiate a cyclic compound from an acyclic compound.
Hence option C is correct.
Note:
For example, the structure of lindane is,
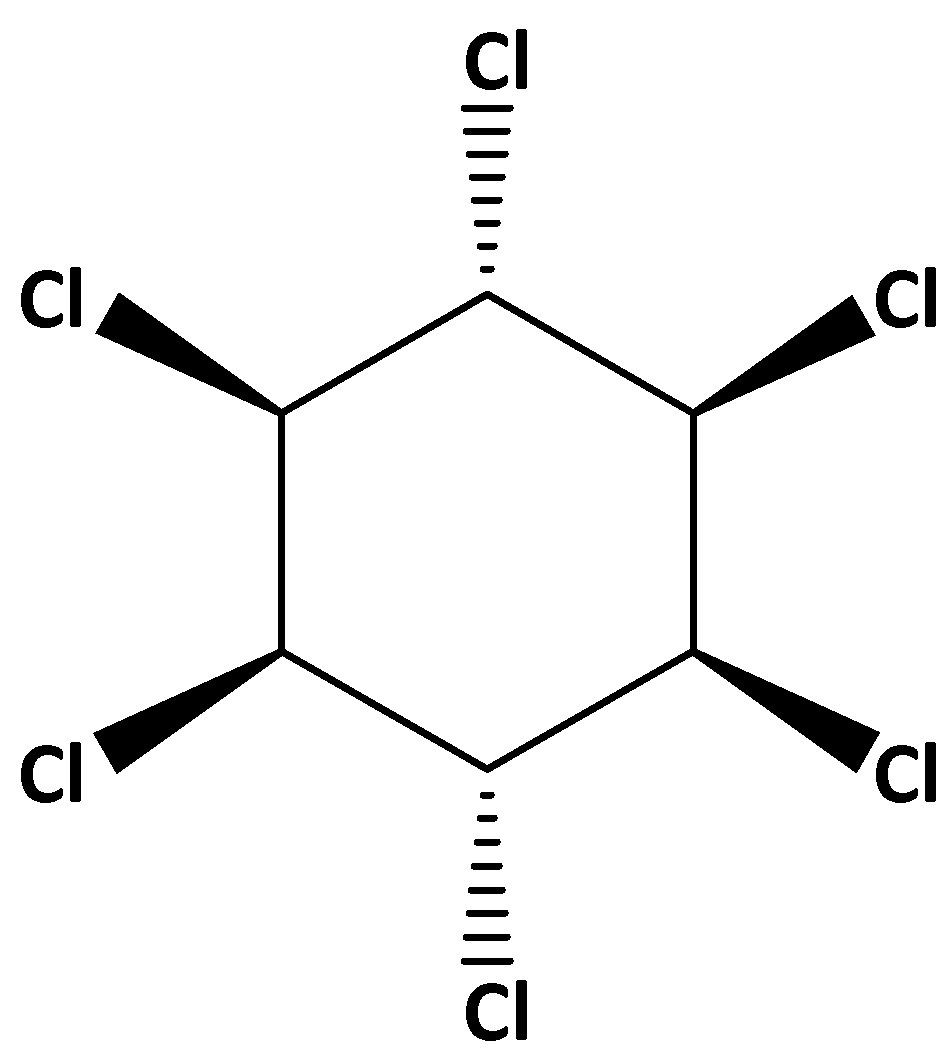
The IUPAC name of lindane is gamma-hexachlorocyclohexane. It is also called benzene hexachloride.
In this structure two chlorine atom which are cis to each other are at gamma position, that is why gamma is written in the IUPAC name.
It is a cyclic compound so “cyclo” prefix is used. Six carbon is present in the ring so “hex” is the word root. And “ane” is used as the primary suffix.
There are six chlorine atoms are present that is why “hexa cholo” is added in the name.
Complete step by step answer:
In IUPAC nomenclature, the root word is basically number of total carbons in a longest chain of that compound.
| No. of carbons | Root word |
| 1 | Meth |
| 2 | Eth |
| 3 | Prop |
| 4 | But |
| 5 | Pent |
… and so on.
The primary suffix is basically used to differentiate between the saturated compounds (Alkanes) and unsaturated compounds (Alkene and Alkynes).
| compound | suffix |
| Alkane | ane |
| Alkene | Ene |
| Alkyne | Yne |
If there are more than one suffix. Then one of those suffixes is considered a secondary suffix.
Example: Methanol (Alkanol) , here ‘ol’ is a secondary suffix.
The primary prefixes are basically used to differentiate between cyclic compound and noncyclic or chain compound. For cyclic compound prefix s ‘cyclo’. if there are any side chains or groups are present then secondary prefixes like ‘methyl’, ‘ethyl’ are used.
So, the primary prefix ’cyclo’ is used in order to differentiate a cyclic compound from an acyclic compound.
Hence option C is correct.
Note:
For example, the structure of lindane is,

The IUPAC name of lindane is gamma-hexachlorocyclohexane. It is also called benzene hexachloride.
In this structure two chlorine atom which are cis to each other are at gamma position, that is why gamma is written in the IUPAC name.
It is a cyclic compound so “cyclo” prefix is used. Six carbon is present in the ring so “hex” is the word root. And “ane” is used as the primary suffix.
There are six chlorine atoms are present that is why “hexa cholo” is added in the name.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




