
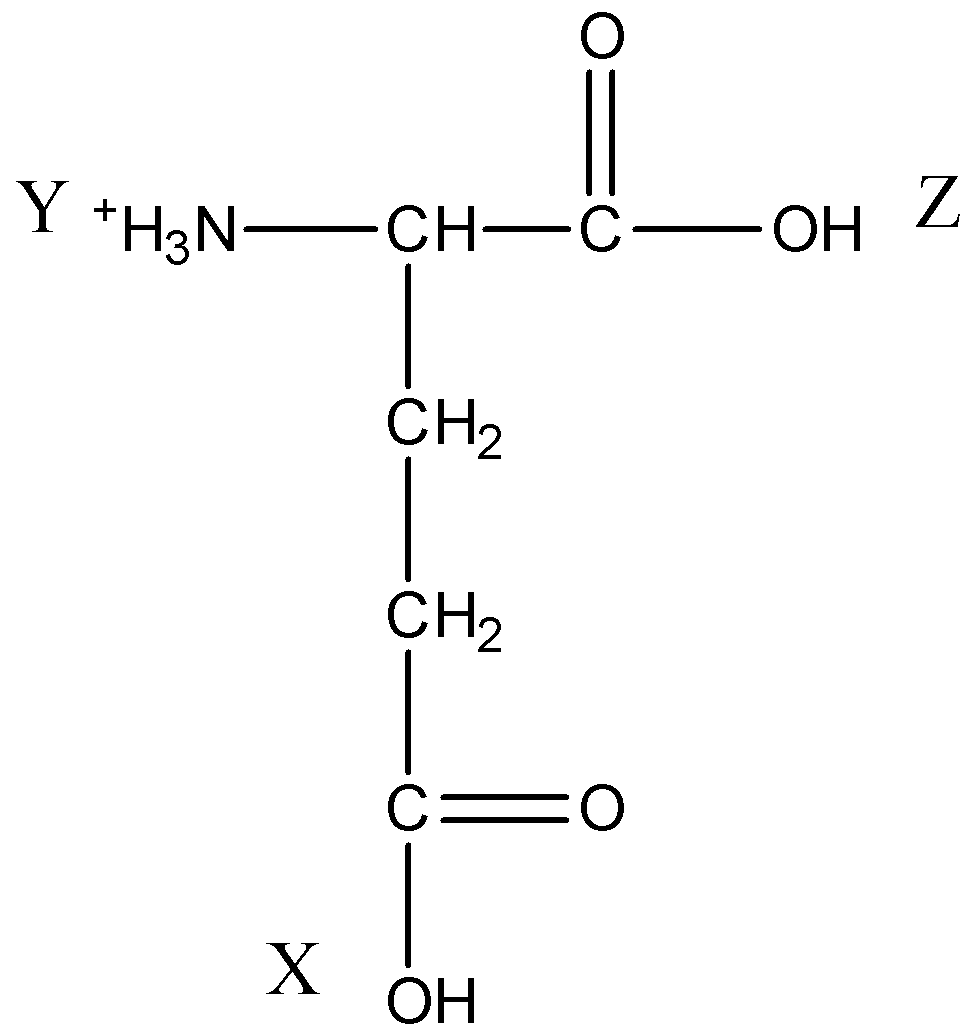
The \[pKa\] values for the three ionizable groups X, Y and Z of glutamic acid 4.3, 9.7 and 2.2 respectively.
The isoelectric point for the amino acid is:
(A)-7.00
(B)-3.25
(C )-4.95
(D)-5.95

Answer
590.4k+ views
Hint: Isoelectric point is at $pH$where molecules are electrically neutral, or it carries no net electrical charge. Amino acids are zwitterions that carry both positive and negative charges in molecules.
To calculate the isoelectric point, first it should be determined whether the amino acid is acidic or basic.
Complete answer:
-Amino acids contain amino and carboxyl acid groups.
-Amino acids can be classified as acidic, basic and neutral depending upon the number of amino or carboxyl groups present in the molecule.
When carboxyl group and amino groups are equal, amino acid is neutral.
When the number of carboxyl groups is more than the amino group present in amino acid, then amino acid is acidic.
When the number of amino groups are more than carboxyl groups present in amino acid, then amino acid is basic.
Amino acids are neutral as they have carboxyl groups which can lose a proton and amino group which can accept protons.
Since there are two carboxyl groups and one amino group in glutamic acid, it is acidic amino acid.
For acidic amino acids, isoelectric point is average of $pKa$ values of carboxyl groups.
So isoelectric point for amino acid is option(B)- 3.25.
Note:
For neutral amino acids, the isoelectric point is 7. Also, by examining it can be concluded that glutamic acid is an acidic amino acid and by observing values given it can be concluded that isoelectric point is between 2.2 and 4.3. Amino acids act as salts as they have both positive and negative charges.
To calculate the isoelectric point, first it should be determined whether the amino acid is acidic or basic.
Complete answer:
-Amino acids contain amino and carboxyl acid groups.
-Amino acids can be classified as acidic, basic and neutral depending upon the number of amino or carboxyl groups present in the molecule.
When carboxyl group and amino groups are equal, amino acid is neutral.
When the number of carboxyl groups is more than the amino group present in amino acid, then amino acid is acidic.
When the number of amino groups are more than carboxyl groups present in amino acid, then amino acid is basic.
Amino acids are neutral as they have carboxyl groups which can lose a proton and amino group which can accept protons.
Since there are two carboxyl groups and one amino group in glutamic acid, it is acidic amino acid.
For acidic amino acids, isoelectric point is average of $pKa$ values of carboxyl groups.
So isoelectric point for amino acid is option(B)- 3.25.
Note:
For neutral amino acids, the isoelectric point is 7. Also, by examining it can be concluded that glutamic acid is an acidic amino acid and by observing values given it can be concluded that isoelectric point is between 2.2 and 4.3. Amino acids act as salts as they have both positive and negative charges.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




