
The pink colour of phenolphthalein in alkaline medium is due to
A. The acidic form of phenolphthalein
B. The anionic form of phenolphthalein
C. $O{{H}^{-}}$of the alkali
D. The non-conjugated structure of phenolphthalein
Answer
595.2k+ views
Hint: To solve this problem, you need to know about the structure of phenolphthalein. Notice what is happening to this structure in the alkaline medium.
Complete step by step solution:
Let us have a look at the structure of phenolphthalein:
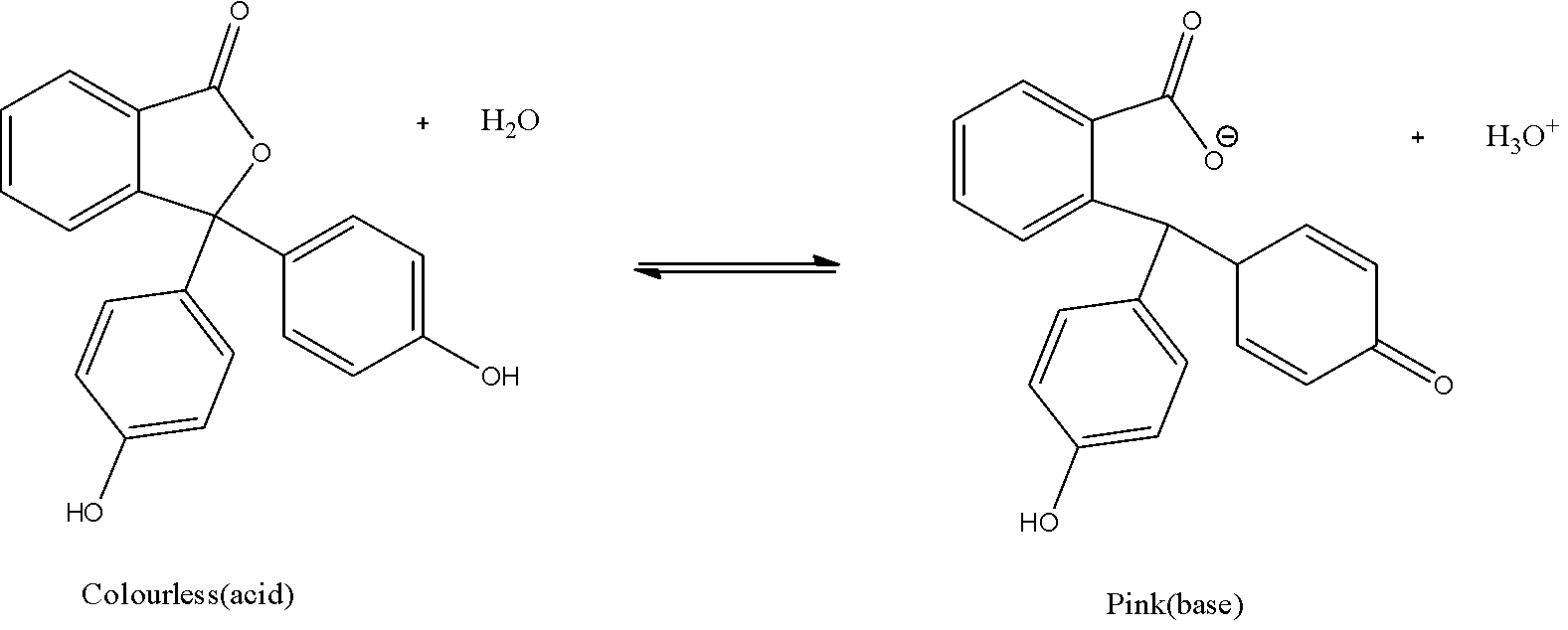
Here, the structure of phenolphthalein in the acidic medium has a ${{H}^{+}}$. While we add a base, the solution becomes basic after the endpoint. In a basic medium, the $O{{H}^{-}}$ions will neutralize this proton and the structure of phenolphthalein becomes an anion. This anionic structure is also known as the quinoid structure is resonance stabilized. This quinoid structure which is an anionic form is responsible for the pink colour in the phenolphthalein.
So, the pink colour of phenolphthalein is due to the anionic form of the phenolphthalein.
So, the correct answer is “Option B”.
Additional Information: - Phenolphthalein’s common use is as an indicator in acid-base titrations. It also serves as a component of universal indicator, together with methyl red, bromothymol blue, and thymol blue.
- Phenolphthalein adopts at least four different states in aqueous solution as a result of pH changes.
- Under strongly acidic conditions, it exists in a protonated form, giving an orange colouration.
- Between strongly acidic and slightly basic conditions, the lactone form is colourless.
- The doubly deprotonated phenolate form gives the familiar pink colour.
- In strongly basic conditions, phenolphthalein is converted to its $ln(O{{H}^{-}})$form, and its pink colour undergoes a rather slow fading reaction and becomes completely colourless above 13.0 pH.
Note: The option ‘A’ is incorrect because the acidic form of phenolphthalein is colourless. Also, the option ‘C’ is incorrect because $O{{H}^{-}}$ does not give any colour to a solution. And the non-conjugated phenolphthalein is not responsible for the colour.
Complete step by step solution:
Let us have a look at the structure of phenolphthalein:

Here, the structure of phenolphthalein in the acidic medium has a ${{H}^{+}}$. While we add a base, the solution becomes basic after the endpoint. In a basic medium, the $O{{H}^{-}}$ions will neutralize this proton and the structure of phenolphthalein becomes an anion. This anionic structure is also known as the quinoid structure is resonance stabilized. This quinoid structure which is an anionic form is responsible for the pink colour in the phenolphthalein.
So, the pink colour of phenolphthalein is due to the anionic form of the phenolphthalein.
So, the correct answer is “Option B”.
Additional Information: - Phenolphthalein’s common use is as an indicator in acid-base titrations. It also serves as a component of universal indicator, together with methyl red, bromothymol blue, and thymol blue.
- Phenolphthalein adopts at least four different states in aqueous solution as a result of pH changes.
- Under strongly acidic conditions, it exists in a protonated form, giving an orange colouration.
- Between strongly acidic and slightly basic conditions, the lactone form is colourless.
- The doubly deprotonated phenolate form gives the familiar pink colour.
- In strongly basic conditions, phenolphthalein is converted to its $ln(O{{H}^{-}})$form, and its pink colour undergoes a rather slow fading reaction and becomes completely colourless above 13.0 pH.
Note: The option ‘A’ is incorrect because the acidic form of phenolphthalein is colourless. Also, the option ‘C’ is incorrect because $O{{H}^{-}}$ does not give any colour to a solution. And the non-conjugated phenolphthalein is not responsible for the colour.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




