
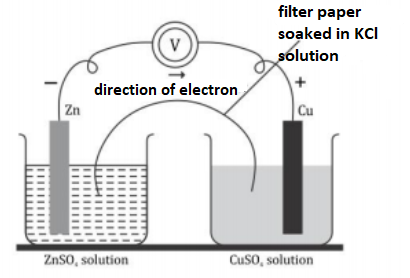
The picture of an electrochemical cell is given.
a.) Identify the anode and cathode of this cell.
b.) Write the chemical reactions taking place in both the electrodes.
c.) Write the redox reaction taking place in the cell

Answer
567.3k+ views
Hint: Electrochemical cell is a device that generates a potential difference between electrodes using chemical reactions.
- Redox reactions are those reactions in which simultaneous oxidation and reduction of the chemical species are involved. In a redox reaction, one substance is reduced, whereas the other is oxidized.
Complete Solution :
a.) In Zn-Cu cell: The flow of electrons in the external circuit from zinc to copper rod. Therefore, the current flows from Copper to zinc. As the electrons are moving from zinc to copper rod, the zinc rod is regarded as a negative terminal and the copper rod is regarded as a positive terminal.
The zinc electrode where oxidation occurs is an anode. Hence, Zinc acts as an anode while the copper electrode where reduction occurs is a cathode. Hence, the copper rod acts as a cathode.
b.) The following chemical reactions will take place:
At cathode: ${ Cu }^{ +2 }{ +2e }^{ - }{ \rightarrow Cu }$
At anode: ${ Zn(s)\rightarrow Zn }^{ +2 }{ +2e }^{ - }$
c.) In this reaction, copper is getting reduced whereas zinc is getting oxidized.
Hence, the redox reaction taking place in the cell is;
${ Zn(s)+Cu }^{ +2 }{ \rightarrow Zn }^{ +2 }{ +Cu }$
Additional Information:
- Oxidation is defined as a process that is characterized by the addition of oxygen or a loss of hydrogen. At the point when a substance picks up oxygen or loses hydrogen during a reaction, it is oxidized.
- The reduction is defined as a process that is characterized by the addition of hydrogen or a loss of oxygen. When a substance loses oxygen or picks up hydrogen during a reaction, it is reduced.
Note: The possibility to make a mistake is that oxidation may occur at the cathode and reduction occurs at the anode but in an electrochemical cell, oxidation occurs at anode and reduction occurs at the cathode.
- Redox reactions are those reactions in which simultaneous oxidation and reduction of the chemical species are involved. In a redox reaction, one substance is reduced, whereas the other is oxidized.
Complete Solution :
a.) In Zn-Cu cell: The flow of electrons in the external circuit from zinc to copper rod. Therefore, the current flows from Copper to zinc. As the electrons are moving from zinc to copper rod, the zinc rod is regarded as a negative terminal and the copper rod is regarded as a positive terminal.
The zinc electrode where oxidation occurs is an anode. Hence, Zinc acts as an anode while the copper electrode where reduction occurs is a cathode. Hence, the copper rod acts as a cathode.
b.) The following chemical reactions will take place:
At cathode: ${ Cu }^{ +2 }{ +2e }^{ - }{ \rightarrow Cu }$
At anode: ${ Zn(s)\rightarrow Zn }^{ +2 }{ +2e }^{ - }$
c.) In this reaction, copper is getting reduced whereas zinc is getting oxidized.
Hence, the redox reaction taking place in the cell is;
${ Zn(s)+Cu }^{ +2 }{ \rightarrow Zn }^{ +2 }{ +Cu }$
Additional Information:
- Oxidation is defined as a process that is characterized by the addition of oxygen or a loss of hydrogen. At the point when a substance picks up oxygen or loses hydrogen during a reaction, it is oxidized.
- The reduction is defined as a process that is characterized by the addition of hydrogen or a loss of oxygen. When a substance loses oxygen or picks up hydrogen during a reaction, it is reduced.
Note: The possibility to make a mistake is that oxidation may occur at the cathode and reduction occurs at the anode but in an electrochemical cell, oxidation occurs at anode and reduction occurs at the cathode.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




