
The phosphate of a certain metal M is ${M_3}{\left( {P{O_4}} \right)_2}.$ The correct formula of metal sulphate would be:
$\left( A \right)\;{M_2}{\left( {S{O_4}} \right)_3}$
$\left( B \right)\;MS{O_4}$
$\left( C \right)\,{M_3}{\left( {S{O_4}} \right)_2}$
$\left( D \right)\,{M_2}S{O_4}$
Answer
570.6k+ views
Hint: In order to solve these questions we must know the oxidation state of cation and anion. Metal has an oxidation state of +2 called cation and phosphate is an anion with oxidation state of –3. Now proceeding according to the given question.
Complete step by step answer:
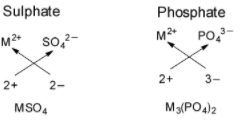
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anion is formed by gain of electrons by non metals. Cations are positively charged ions formed when neutral atoms lose electrons; anions are negatively charged ions formed when neutral atoms gain electrons. Ionic compounds must be electrically neutral.
In ${M_3}{\left( {P{O_4}} \right)_2},$ metal is having an oxidation state of +2 called as ${M^{2 + }}$ cation and phosphate $PO_4^{3 - }$ cation and phosphate $PO_4^{3 - }$ is an anion with oxidation state of –3. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios.
For formation of sulphate, the metal with oxidation state of +2 would be combining with sulphate $SO_4^{2 - }$ is an anion with oxidation state of –2 to give $MS{O_4}.$
Hence, the correct answer will be option (B) $MS{O_4}.$
Note:
These types of questions depend upon the valency of an element. An element's valence is the number of hydrogen atoms which can combine with or replace (directly or indirectly) one of the element’s atoms. If the number of electrons in the outer shell is between 1 to 4, the compound is said to have positive valency. For compounds with electrons four, five, six, or seven, the valency is determined by subtracting the electrons from eight.
Complete step by step answer:
For formation of a neutral ionic compound, the charges on cation and anion must be balanced. The cation is formed by loss of electrons by metals and anion is formed by gain of electrons by non metals. Cations are positively charged ions formed when neutral atoms lose electrons; anions are negatively charged ions formed when neutral atoms gain electrons. Ionic compounds must be electrically neutral.
In ${M_3}{\left( {P{O_4}} \right)_2},$ metal is having an oxidation state of +2 called as ${M^{2 + }}$ cation and phosphate $PO_4^{3 - }$ cation and phosphate $PO_4^{3 - }$ is an anion with oxidation state of –3. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios.
For formation of sulphate, the metal with oxidation state of +2 would be combining with sulphate $SO_4^{2 - }$ is an anion with oxidation state of –2 to give $MS{O_4}.$

Hence, the correct answer will be option (B) $MS{O_4}.$
Note:
These types of questions depend upon the valency of an element. An element's valence is the number of hydrogen atoms which can combine with or replace (directly or indirectly) one of the element’s atoms. If the number of electrons in the outer shell is between 1 to 4, the compound is said to have positive valency. For compounds with electrons four, five, six, or seven, the valency is determined by subtracting the electrons from eight.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




