
The pH of a dilute solution of acetic acid was found to be 4.3. The addition of a small crystal of sodium acetate will cause pH to:
Answer
588.9k+ views
Hint: Write the reaction for dissolution of acetic acid in water to find the ions after reaction. Now write the equilibrium constant. The pH of solution depends on the concentration of hydrogen ions. When sodium acetate is added to this solution, the concentration of acetate ions increases as sodium acetate as it is easily soluble. This will affect the equilibrium of dissolution of acetic acid. Since the products are in excess, the reaction will go backwards, thereby reducing dissolution.
Complete step-by-step answer:
Organic acids are in general considered to be weak acids due to relatively less stable conjugate base in comparison to a mineral acid.
The structure of ethanoic acid is :
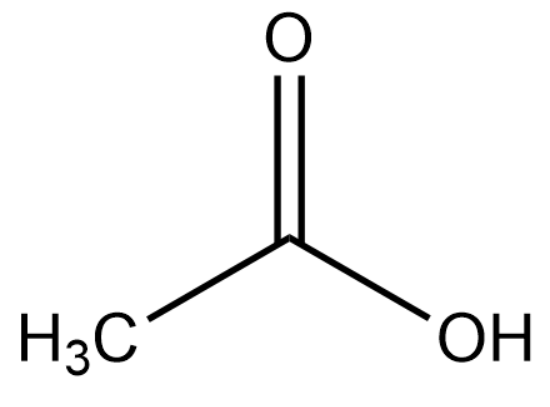
Upon deprotonation, the conjugate base of ethanoic acid becomes:
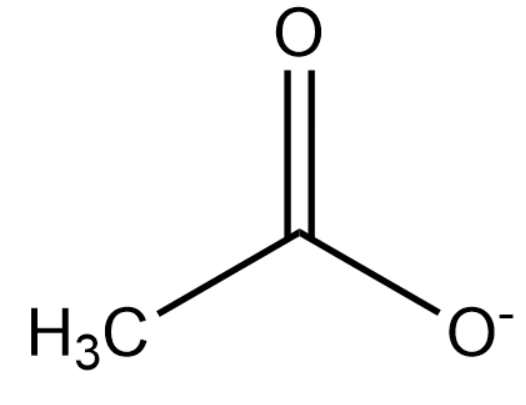
In the above structure, there is resonance between the two oxygen atoms attached to the carbon atom which stabilises the negative charge. However, the methyl group increases electron density on the carbon atom making the resonating structure unstable. This is the reason why ethanoic acid is considered to be a weak acid.
We will now write the dissolution of acetic acid and sodium acetate in water.
$\text{C}{{\text{H}}_{\text{3}}}\text{COOH }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + }{{\text{H}}^{+}}$
$\text{C}{{\text{H}}_{\text{3}}}\text{COONa }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + N}{{\text{a}}^{+}}$
Sodium acetate dissolves much faster than acetic acid in water. Due to this the concentration of acetate ions increases in the products side.
Due to this the dissolution of acetic acid is moved backwards. The concentration hydrogen ions decrease. As a result of this pH of the solution increases making the solution less acidic.
From the above explanation we can conclude that the addition of a small crystal of sodium acetate will cause pH to become more than 4.3.
Therefore, the correct answer is option (B).
Note: In the above reaction we see that the dissolution of acetic acid is reduced due to addition of sodium acetate. In the same way if we remove the acetate ions being formed in the product side, the reaction moves forward thereby increasing the concentration of hydrogen ions in the product side. In this way the pH of the solution decreases making the solution more acidic.
Complete step-by-step answer:
Organic acids are in general considered to be weak acids due to relatively less stable conjugate base in comparison to a mineral acid.
The structure of ethanoic acid is :

Upon deprotonation, the conjugate base of ethanoic acid becomes:

In the above structure, there is resonance between the two oxygen atoms attached to the carbon atom which stabilises the negative charge. However, the methyl group increases electron density on the carbon atom making the resonating structure unstable. This is the reason why ethanoic acid is considered to be a weak acid.
We will now write the dissolution of acetic acid and sodium acetate in water.
$\text{C}{{\text{H}}_{\text{3}}}\text{COOH }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + }{{\text{H}}^{+}}$
$\text{C}{{\text{H}}_{\text{3}}}\text{COONa }\to \text{ C}{{\text{H}}_{\text{3}}}\text{CO}{{\text{O}}^{-}}\text{ + N}{{\text{a}}^{+}}$
Sodium acetate dissolves much faster than acetic acid in water. Due to this the concentration of acetate ions increases in the products side.
Due to this the dissolution of acetic acid is moved backwards. The concentration hydrogen ions decrease. As a result of this pH of the solution increases making the solution less acidic.
From the above explanation we can conclude that the addition of a small crystal of sodium acetate will cause pH to become more than 4.3.
Therefore, the correct answer is option (B).
Note: In the above reaction we see that the dissolution of acetic acid is reduced due to addition of sodium acetate. In the same way if we remove the acetate ions being formed in the product side, the reaction moves forward thereby increasing the concentration of hydrogen ions in the product side. In this way the pH of the solution decreases making the solution more acidic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




