
The percentage s-character of the hybrid orbitals in methane, ethene and ethyne are respectively:
(A) 25, 33, 50
(B) 25, 50, 75
(C) 50, 75, 100
(D) 10, 20, 40
Answer
594.9k+ views
Hint: Methane has 1 carbon atom attached to 4 H atoms giving it a tetrahedral shape. While ethene has a double bond between both C atoms and ethyne has a triple bond. Now find their hybridisation. The type of hybridisation will itself give you the percentage s-character.
Formula used: Formula for finding out the hybridization of any molecule:
$H = 1/2\left[ {V + M - C + A} \right]$
Where, V = number of valence electrons;
M = monovalent atoms;
C = positive charge;
A = negative charge.
Complete step by step answer:
First we need to find out the hybridisation of all the 3 compounds.
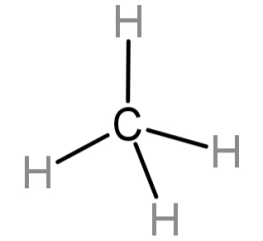
-Let’s begin with methane. Methane has the molecular formula of: $C{H_4}$. In this the carbon atom has 4 electrons that are available for bonding and all 4 of them are bonded to a H atom each. So, the hybridisation will be $s{p^3}$. Even though by imagining the structure we get the hybridisation we can find it out using the formula also.
Using above given formula we can find the hybridisation: $H = 1/2\left[ {V + M - C + A} \right]$
For $C{H_4}$: V = 4, M = 4, C = 0, A = 0.
H = $\frac{1}{2}\left[ {4 + 4 + 0 + 0} \right]$
= 4
Since the hybridisation number is 4 so the hybridisation will be $s{p^3}$. The hybridisation type $s{p^3}$ has 25 % s character.
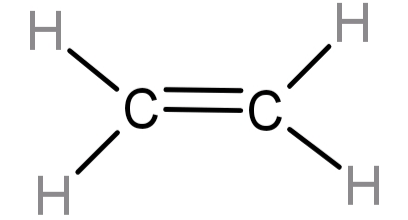
-For ethene: It’s molecular formula is: ${C_2}{H_4}$. The 2 C atoms are connected to each other by a double bond (1 sigma bond and 1 pi bond) and each carbon is also attached to 2 H atoms. This means that 1 atom of C has 3 bonding domains and hence its hybridisation number will be 3. So, it has $s{p^2}$ type hybridisation. For this the percentage character is 33%.
-For ethyne: It’s molecular formula is ${C_2}{H_2}$. In this both the carbons are bonded to each other by a triple bond (1 sigma bond and 2 pi bonds) and each carbon is also bonded with 1 H atom each through a sigma bond. This means that each carbon atom has 2 bonding domains and hence its hybridisation is sp. For this the percentage s- character is 50%.

So, the correct option is: (A) 25, 33, 50
Note: Remember that sp has 50% s character because we are talking about (1s+ 1p) orbitals, $s{p^2}$ has 33% s-character due to (1s+2p) orbitals and in the same way $s{p^3}$has 25% s-character. The same way it goes on for others.
Formula used: Formula for finding out the hybridization of any molecule:
$H = 1/2\left[ {V + M - C + A} \right]$
Where, V = number of valence electrons;
M = monovalent atoms;
C = positive charge;
A = negative charge.
Complete step by step answer:
First we need to find out the hybridisation of all the 3 compounds.
-Let’s begin with methane. Methane has the molecular formula of: $C{H_4}$. In this the carbon atom has 4 electrons that are available for bonding and all 4 of them are bonded to a H atom each. So, the hybridisation will be $s{p^3}$. Even though by imagining the structure we get the hybridisation we can find it out using the formula also.
Using above given formula we can find the hybridisation: $H = 1/2\left[ {V + M - C + A} \right]$
For $C{H_4}$: V = 4, M = 4, C = 0, A = 0.
H = $\frac{1}{2}\left[ {4 + 4 + 0 + 0} \right]$
= 4
Since the hybridisation number is 4 so the hybridisation will be $s{p^3}$. The hybridisation type $s{p^3}$ has 25 % s character.

-For ethene: It’s molecular formula is: ${C_2}{H_4}$. The 2 C atoms are connected to each other by a double bond (1 sigma bond and 1 pi bond) and each carbon is also attached to 2 H atoms. This means that 1 atom of C has 3 bonding domains and hence its hybridisation number will be 3. So, it has $s{p^2}$ type hybridisation. For this the percentage character is 33%.

-For ethyne: It’s molecular formula is ${C_2}{H_2}$. In this both the carbons are bonded to each other by a triple bond (1 sigma bond and 2 pi bonds) and each carbon is also bonded with 1 H atom each through a sigma bond. This means that each carbon atom has 2 bonding domains and hence its hybridisation is sp. For this the percentage s- character is 50%.

So, the correct option is: (A) 25, 33, 50
Note: Remember that sp has 50% s character because we are talking about (1s+ 1p) orbitals, $s{p^2}$ has 33% s-character due to (1s+2p) orbitals and in the same way $s{p^3}$has 25% s-character. The same way it goes on for others.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




