
The pair of species with similar shape is
(A)${\rm{PC}}{{\rm{l}}_3}$, ${\rm{N}}{{\rm{H}}_{\rm{3}}}$
(B) ${\rm{C}}{{\rm{F}}_{\rm{4}}}$, ${\rm{S}}{{\rm{F}}_{\rm{4}}}$
(C) ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$,${\rm{C}}{{\rm{O}}_{\rm{2}}}$
(D) ${\rm{P}}{{\rm{F}}_{\rm{5}}}$, ${\rm{I}}{{\rm{F}}_{\rm{5}}}$
Answer
575.7k+ views
Hint: We know that valence shell electron pair repulsion (VSEPR) theory is used to determine the geometry and shape of molecules from the electron pairs surrounding the central atom. The electron pairs may be bonded pairs or lone pairs.
Complete step by step answer:
Let’s find the correct answer from the given options.
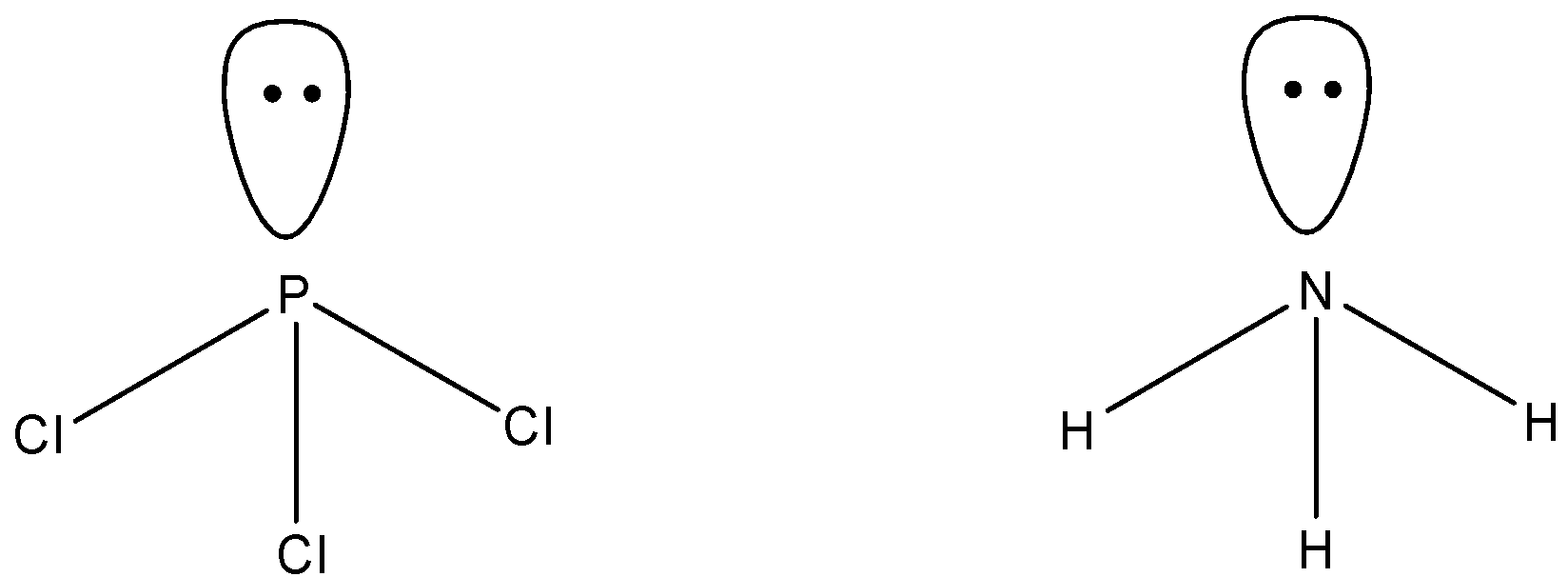
Option A says ${\rm{PC}}{{\rm{l}}_3}$ and ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ has similar shape. Now, we have to determine if they have similar shapes or not.
Phosphorus and nitrogen both belong to group 15 of the periodic table. So, valence electrons in both the elements are 5. In ${\rm{PC}}{{\rm{l}}_3}$, three electrons of nitrogen are shared with three chlorine atoms. So, the number of bond pairs in ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ is 3 and an unshared electron pair present. Similarly in ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ also, three bond pairs and one lone pair present. If a compound has three bond pairs and one pair, the shape is always pyramidal. So, the shape of both compounds is similar, that is pyramidal.
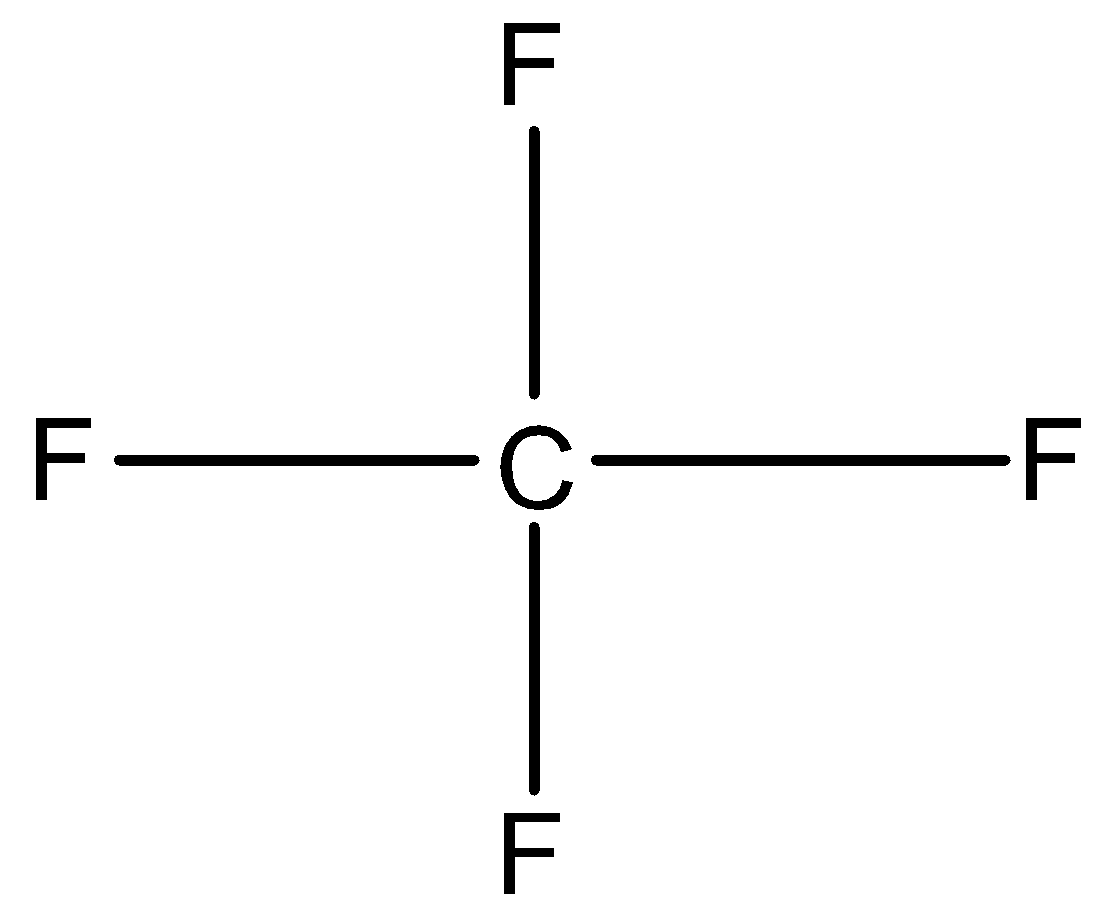
Option B is ${\rm{C}}{{\rm{F}}_{\rm{4}}}$ and${\rm{S}}{{\rm{F}}_{\rm{4}}}$. In ${\rm{C}}{{\rm{F}}_{\rm{4}}}$, Carbon has four valence electrons. So, it shares its four electrons with four fluorine atoms. So, four bond pairs present in ${\rm{C}}{{\rm{F}}_{\rm{4}}}$. Therefore, the structure of ${\rm{C}}{{\rm{F}}_{\rm{4}}}$is tetrahedral.
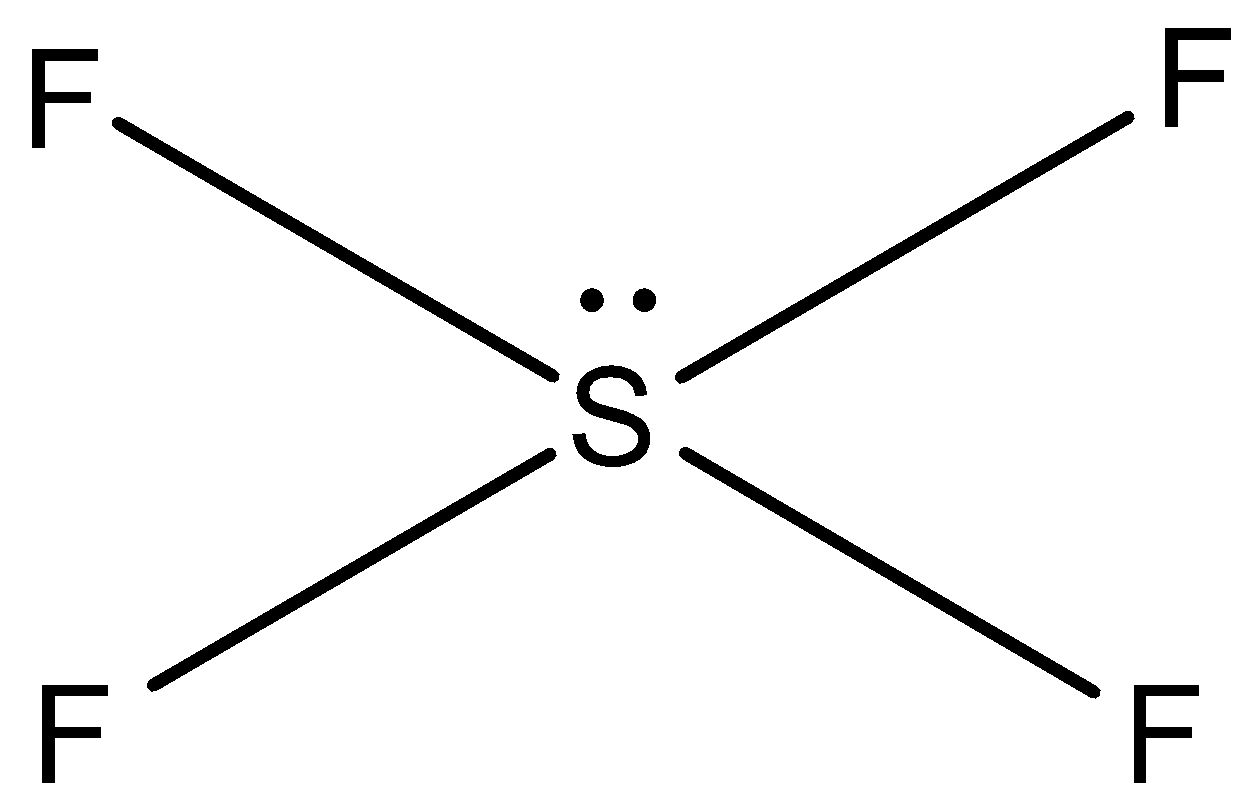
In ${\rm{S}}{{\rm{F}}_{\rm{4}}}$, sulphur has six valence electrons and it forms only four bonds with four fluorine atoms. Two electrons are unshared in the compound (lone pair). So, four lone pairs and one pair is present in ${\rm{S}}{{\rm{F}}_{\rm{4}}}$. So, the shape of ${\rm{S}}{{\rm{F}}_{\rm{4}}}$ is see-saw.
Therefore, both the compounds do not possess similar shape.
Option C is ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$ and${\rm{C}}{{\rm{O}}_{\rm{2}}}$.
In ${\rm{C}}{{\rm{O}}_{\rm{2}}}$, four valence electrons of carbon are shared with two oxygen atoms. So, the compound forms two double bonds. That means, electrons groups surrounding carbon atoms are two. Therefore the structure of ${\rm{C}}{{\rm{O}}_{\rm{2}}}$ is linear.
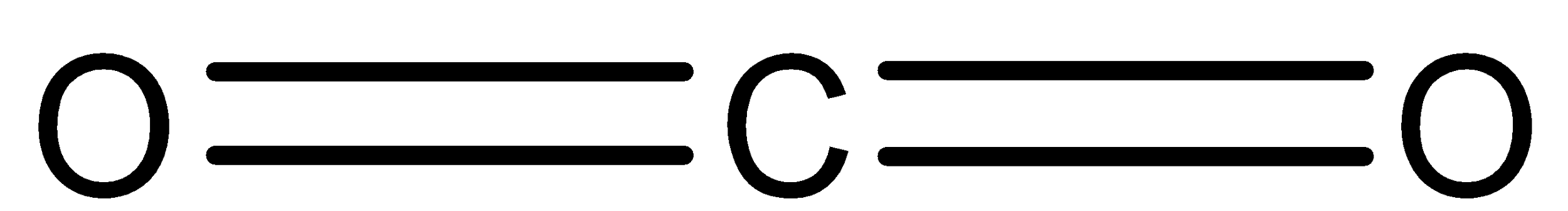
In ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$, Pb has four valence electrons and chlorine has valency of one. So, two electrons of Pb are shared with two chlorine atoms to form two bond pairs. And one pair of electrons is unshared. So, the structure of ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$ is bent.
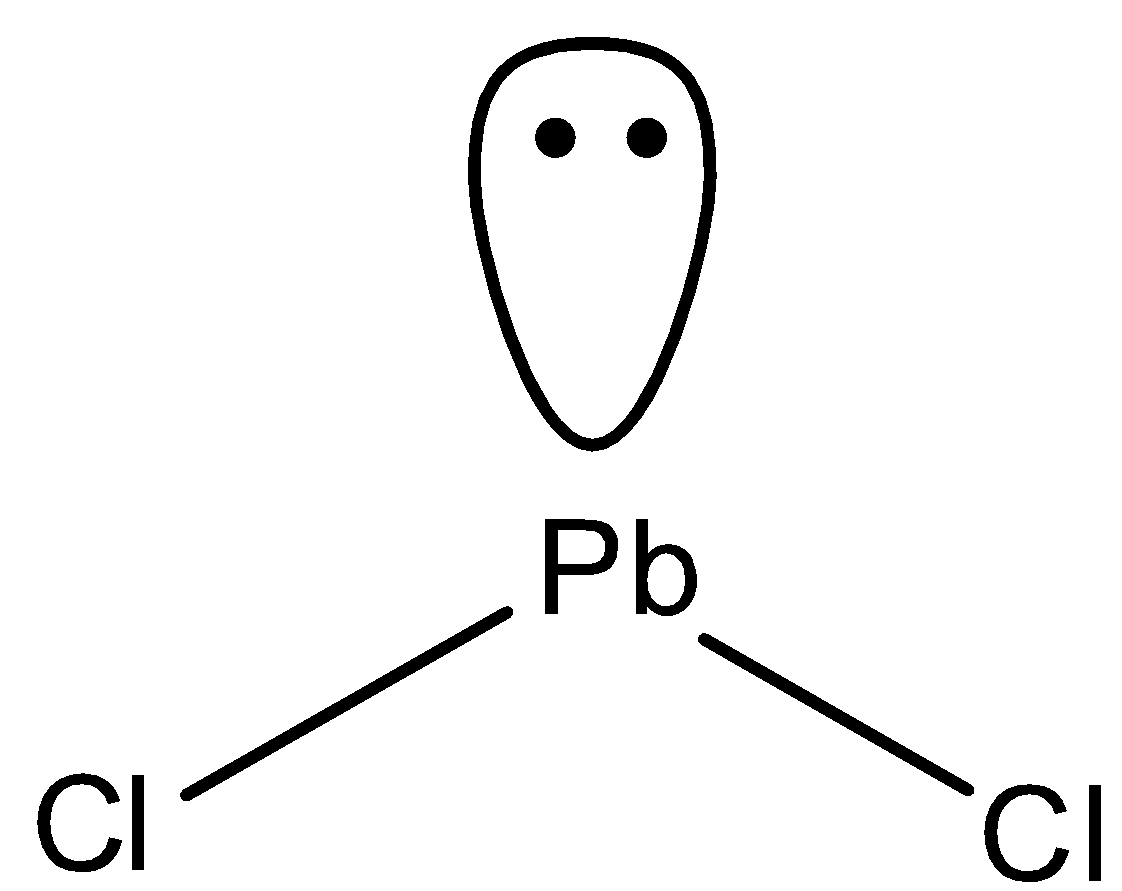
So, both ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$ and${\rm{C}}{{\rm{O}}_{\rm{2}}}$ possess different structures.
OptionD is ${\rm{P}}{{\rm{F}}_{\rm{5}}}$ and ${\rm{I}}{{\rm{F}}_{\rm{5}}}$. In ${\rm{P}}{{\rm{F}}_{\rm{5}}}$, phosphorus has five valence and chlorine has valency one. So, five electrons of phosphorus are shared with five fluorine atoms. So, no lone pair is present. Therefore, the shape of ${\rm{P}}{{\rm{F}}_{\rm{5}}}$ is trigonal bipyramidal.
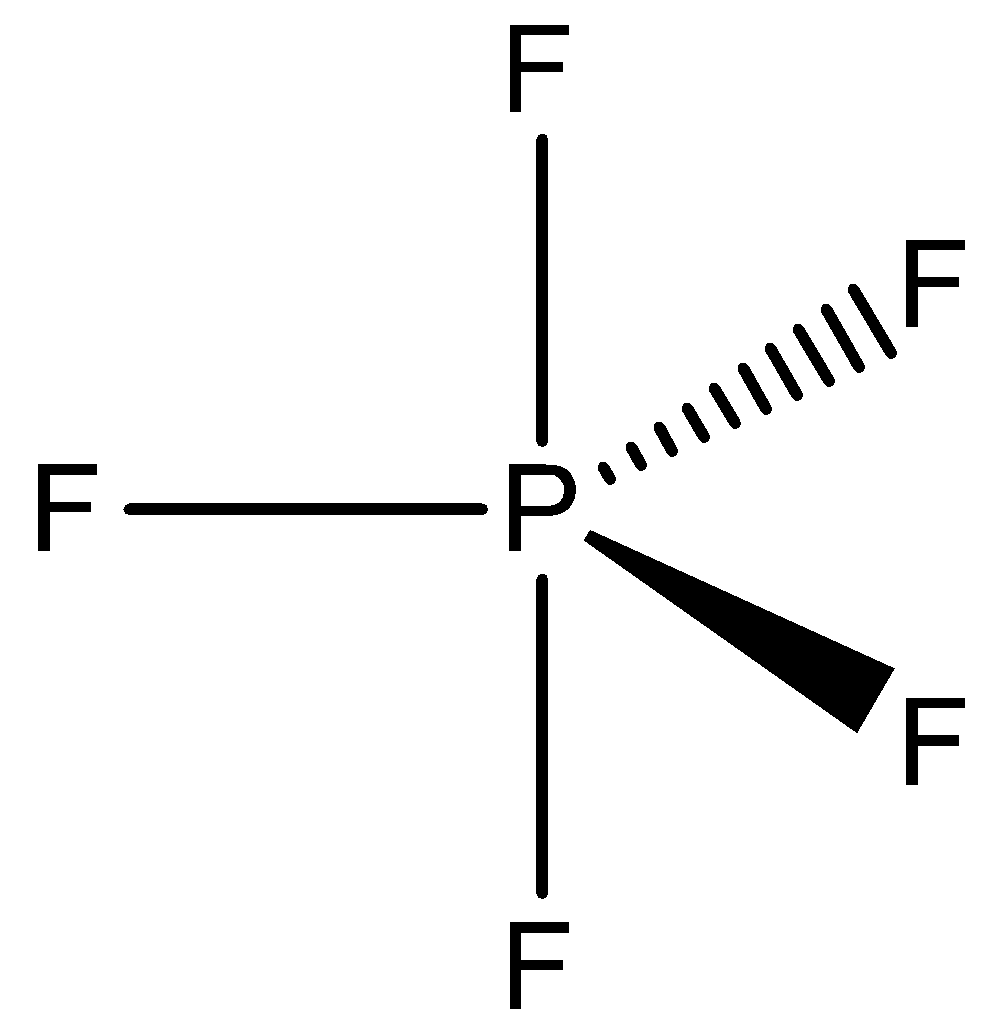
In ${\rm{I}}{{\rm{F}}_{\rm{5}}}$, iodine has seven electrons and fluorine is one. So, five electrons of iodine are shared with five fluorine atoms and one electron pair is unshared. So, in the compound five bond pairs and one lone pair are present. Therefore, the shape of ${\rm{I}}{{\rm{F}}_{\rm{5}}}$is square pyramidal.
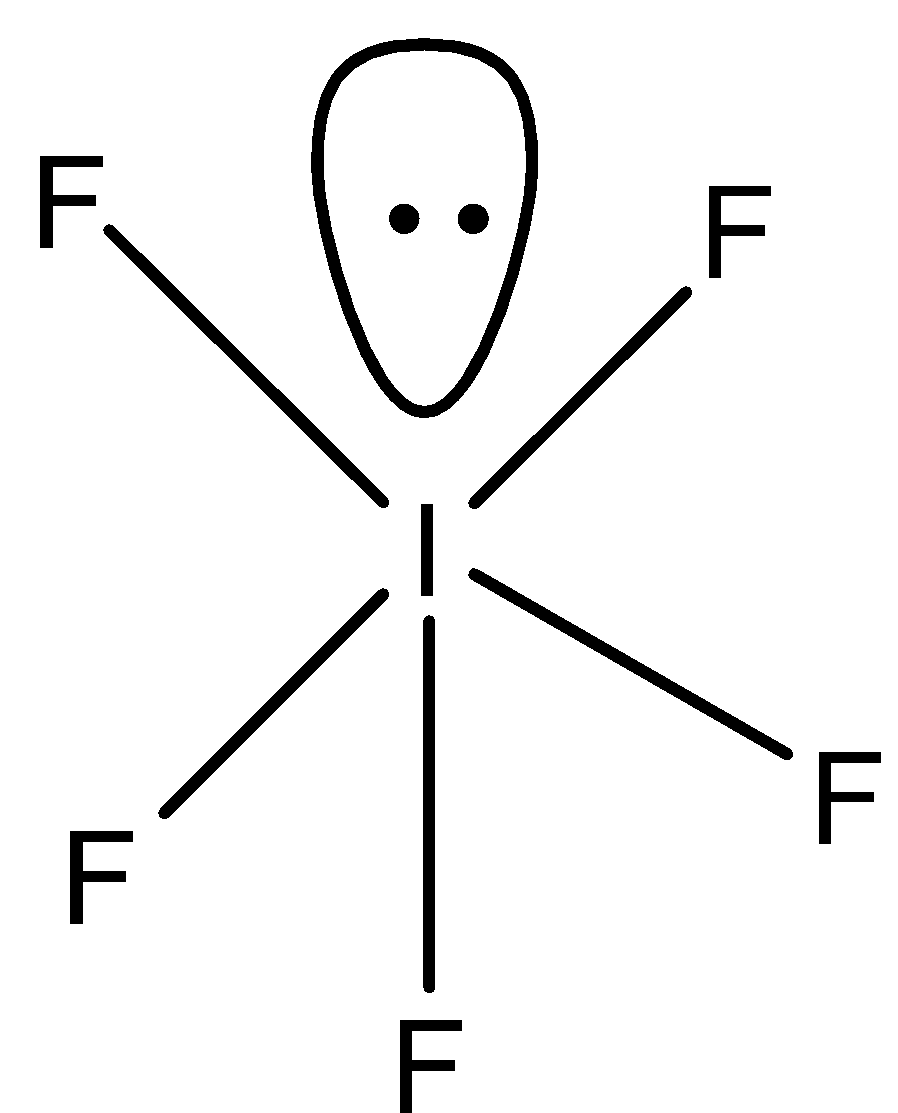
Therefore, both the compounds have different shapes.
So, the correct answer is Option A.
Note: According to VSEPR theory, lone pair electrons repel each other more strongly than that of bond pair electrons. So, the decreasing order of repulsion is lp-lp>lp-bp>bp-bp. So, repulsion between bond pair-bond pair is least and between lone pair-lone pair is highest.
Complete step by step answer:
Let’s find the correct answer from the given options.
Option A says ${\rm{PC}}{{\rm{l}}_3}$ and ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ has similar shape. Now, we have to determine if they have similar shapes or not.
Phosphorus and nitrogen both belong to group 15 of the periodic table. So, valence electrons in both the elements are 5. In ${\rm{PC}}{{\rm{l}}_3}$, three electrons of nitrogen are shared with three chlorine atoms. So, the number of bond pairs in ${\rm{PC}}{{\rm{l}}_{\rm{5}}}$ is 3 and an unshared electron pair present. Similarly in ${\rm{N}}{{\rm{H}}_{\rm{3}}}$ also, three bond pairs and one lone pair present. If a compound has three bond pairs and one pair, the shape is always pyramidal. So, the shape of both compounds is similar, that is pyramidal.

Option B is ${\rm{C}}{{\rm{F}}_{\rm{4}}}$ and${\rm{S}}{{\rm{F}}_{\rm{4}}}$. In ${\rm{C}}{{\rm{F}}_{\rm{4}}}$, Carbon has four valence electrons. So, it shares its four electrons with four fluorine atoms. So, four bond pairs present in ${\rm{C}}{{\rm{F}}_{\rm{4}}}$. Therefore, the structure of ${\rm{C}}{{\rm{F}}_{\rm{4}}}$is tetrahedral.

In ${\rm{S}}{{\rm{F}}_{\rm{4}}}$, sulphur has six valence electrons and it forms only four bonds with four fluorine atoms. Two electrons are unshared in the compound (lone pair). So, four lone pairs and one pair is present in ${\rm{S}}{{\rm{F}}_{\rm{4}}}$. So, the shape of ${\rm{S}}{{\rm{F}}_{\rm{4}}}$ is see-saw.

Therefore, both the compounds do not possess similar shape.
Option C is ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$ and${\rm{C}}{{\rm{O}}_{\rm{2}}}$.
In ${\rm{C}}{{\rm{O}}_{\rm{2}}}$, four valence electrons of carbon are shared with two oxygen atoms. So, the compound forms two double bonds. That means, electrons groups surrounding carbon atoms are two. Therefore the structure of ${\rm{C}}{{\rm{O}}_{\rm{2}}}$ is linear.
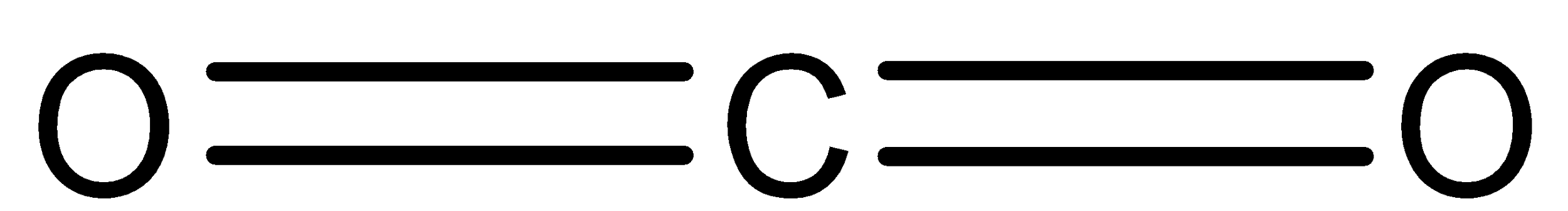
In ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$, Pb has four valence electrons and chlorine has valency of one. So, two electrons of Pb are shared with two chlorine atoms to form two bond pairs. And one pair of electrons is unshared. So, the structure of ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$ is bent.

So, both ${\rm{PbC}}{{\rm{l}}_{\rm{2}}}$ and${\rm{C}}{{\rm{O}}_{\rm{2}}}$ possess different structures.
OptionD is ${\rm{P}}{{\rm{F}}_{\rm{5}}}$ and ${\rm{I}}{{\rm{F}}_{\rm{5}}}$. In ${\rm{P}}{{\rm{F}}_{\rm{5}}}$, phosphorus has five valence and chlorine has valency one. So, five electrons of phosphorus are shared with five fluorine atoms. So, no lone pair is present. Therefore, the shape of ${\rm{P}}{{\rm{F}}_{\rm{5}}}$ is trigonal bipyramidal.

In ${\rm{I}}{{\rm{F}}_{\rm{5}}}$, iodine has seven electrons and fluorine is one. So, five electrons of iodine are shared with five fluorine atoms and one electron pair is unshared. So, in the compound five bond pairs and one lone pair are present. Therefore, the shape of ${\rm{I}}{{\rm{F}}_{\rm{5}}}$is square pyramidal.

Therefore, both the compounds have different shapes.
So, the correct answer is Option A.
Note: According to VSEPR theory, lone pair electrons repel each other more strongly than that of bond pair electrons. So, the decreasing order of repulsion is lp-lp>lp-bp>bp-bp. So, repulsion between bond pair-bond pair is least and between lone pair-lone pair is highest.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




