
The pair of compounds which can form a coordinate bond is:
A.\[{\left( {{C_2}{H_5}} \right)_3}B\] and \[{\left( {C{H_3}} \right)_3}N\]
B.\[HCl\,and\,{H_2}\]
C.\[B{F_3}\] and \[N{H_3}\]
D.\[(1)\] and $(3)$ both
Answer
478.5k+ views
Hint: We have to know that the coordinate bond is a type of covalent bond and it is established from a single atom by sharing the pair of electrons. And each pair of electrons are contributed by the same atom. And this coordinate bond is also known as dipolar bond. After the sharing of electrons in a coordinate bond, the atom will become more stable. And the atom which shares the electron which acts as donor.
Complete answer:
\[{\left( {{C_2}{H_5}} \right)_3}B\] and \[{\left( {C{H_3}} \right)_3}N\] Pair of compounds which can form a coordinate bond. But these pairs of compounds are not the only ones forming coordinate bonds. Hence, option (A) is incorrect.
The pair of hydrochloric acid and hydrogen does not form a coordinate bond. Hence, the option (B) is incorrect.
\[B{F_3}\] and \[N{H_3}\] Pair of compounds which can form a coordinate bond. But \[{\left( {{C_2}{H_5}} \right)_3}B\] and \[{\left( {C{H_3}} \right)_3}N\] is also form the coordinate bond. Hence, option (C) is incorrect.
Among these options, \[{\left( {{C_2}{H_5}} \right)_3}B\] and \[{\left( {C{H_3}} \right)_3}N\] and \[B{F_3}\] and \[N{H_3}\] pairs of compounds which can form a coordinate bond. Because, here the triethyl borane and triethylamine are making a bond by the sharing of electrons which is present in nitrogen and boron. One empty p-orbital is present in boron. And it can be represented as,
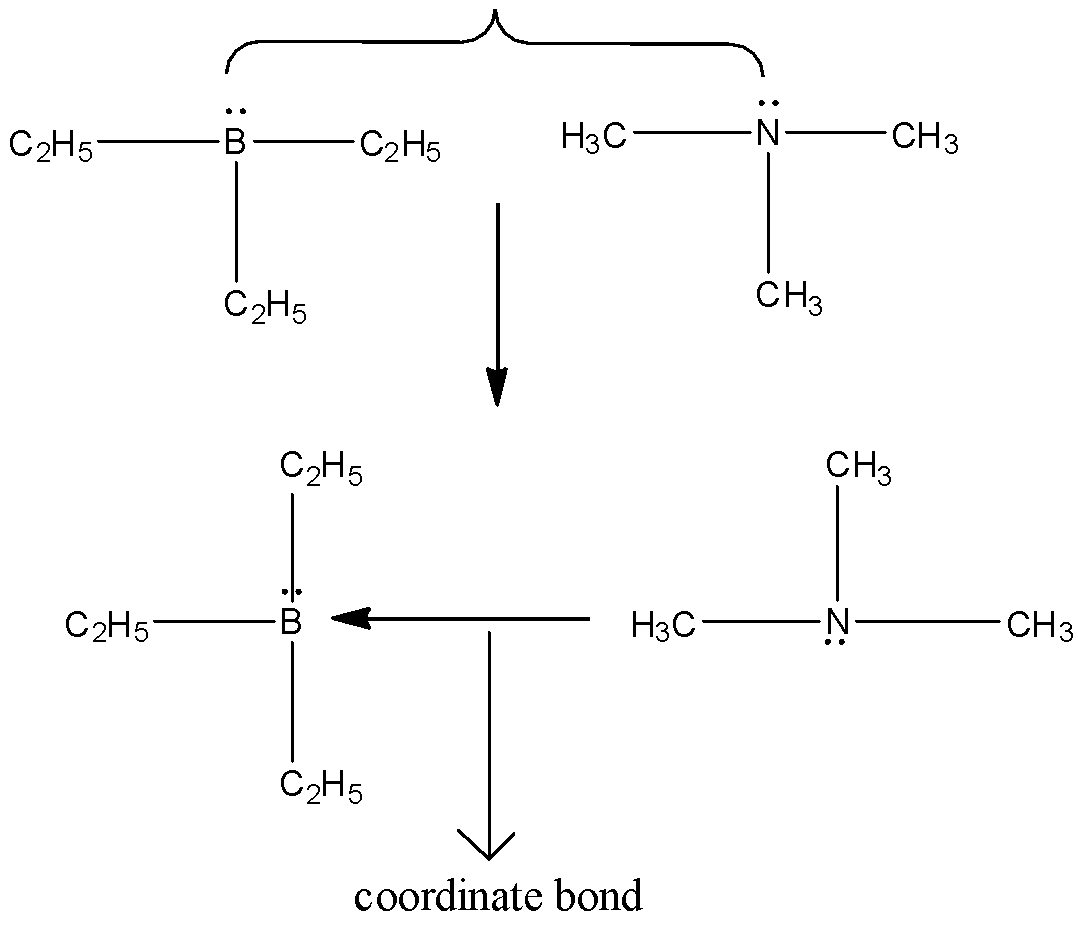
And in the case of ammonia and boron trifluoride can represented as,
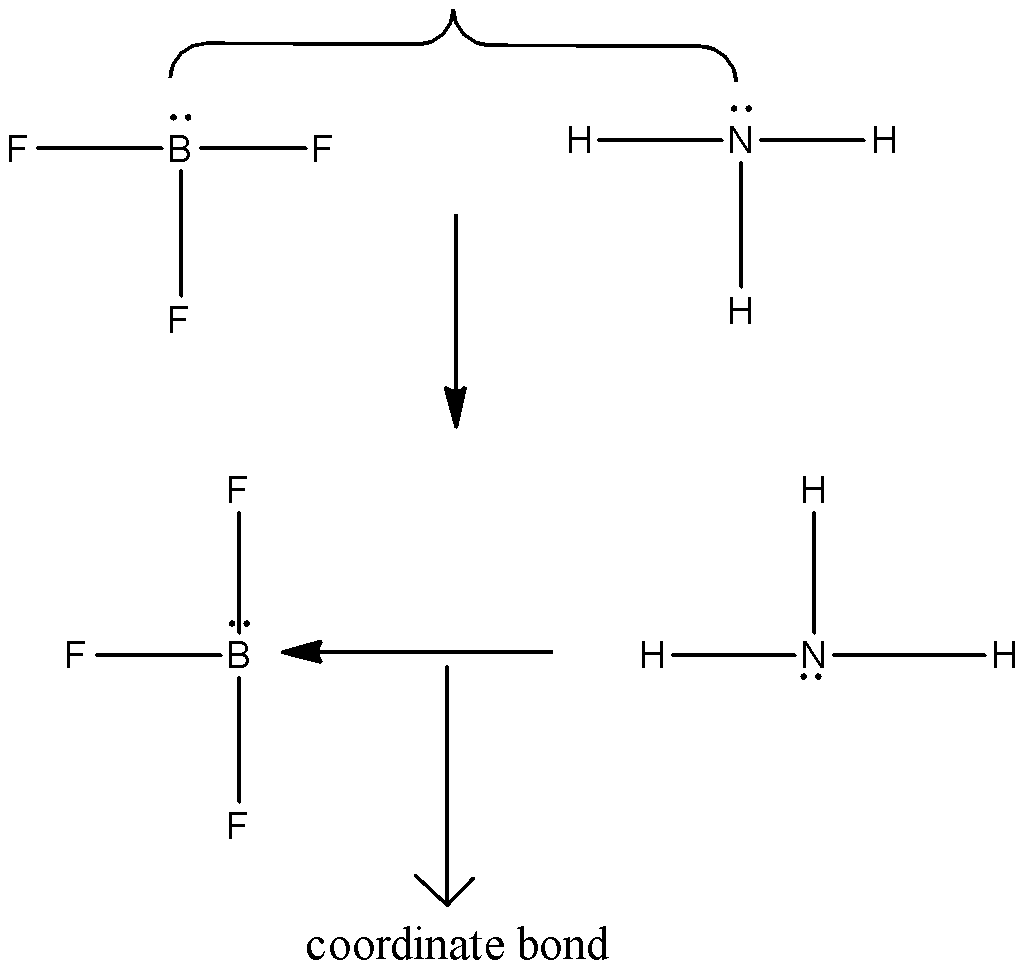
Therefore, a pair of compounds which can form a coordinate bond.
Hence, the option (D) is correct.
Note:
Among these given compounds, triethyl borane – triethylamine pair and boron trifluoride – ammonia pair will form the coordinate bond. Because, each pair of central atoms have unpaired electrons and there is a formation of covalent bond by sharing this pair of electrons. And the pairing of electrons is attached by both nuclei present in the central atom.
Complete answer:
\[{\left( {{C_2}{H_5}} \right)_3}B\] and \[{\left( {C{H_3}} \right)_3}N\] Pair of compounds which can form a coordinate bond. But these pairs of compounds are not the only ones forming coordinate bonds. Hence, option (A) is incorrect.
The pair of hydrochloric acid and hydrogen does not form a coordinate bond. Hence, the option (B) is incorrect.
\[B{F_3}\] and \[N{H_3}\] Pair of compounds which can form a coordinate bond. But \[{\left( {{C_2}{H_5}} \right)_3}B\] and \[{\left( {C{H_3}} \right)_3}N\] is also form the coordinate bond. Hence, option (C) is incorrect.
Among these options, \[{\left( {{C_2}{H_5}} \right)_3}B\] and \[{\left( {C{H_3}} \right)_3}N\] and \[B{F_3}\] and \[N{H_3}\] pairs of compounds which can form a coordinate bond. Because, here the triethyl borane and triethylamine are making a bond by the sharing of electrons which is present in nitrogen and boron. One empty p-orbital is present in boron. And it can be represented as,

And in the case of ammonia and boron trifluoride can represented as,

Therefore, a pair of compounds which can form a coordinate bond.
Hence, the option (D) is correct.
Note:
Among these given compounds, triethyl borane – triethylamine pair and boron trifluoride – ammonia pair will form the coordinate bond. Because, each pair of central atoms have unpaired electrons and there is a formation of covalent bond by sharing this pair of electrons. And the pairing of electrons is attached by both nuclei present in the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




