
The packing fraction of a body-centered cube is:
a.) ${ 0.42 }$
b.) ${ 0.53 }$
c.) ${ 0.68 }$
d.) ${ 0.82 }$
Answer
572.1k+ views
Hint: Before solving the question let's understand the term packing fraction. Packing fraction or packing efficiency = The volume occupied by the spheres divided by the volume of a cube is known as packing fraction/efficiency.
Complete Solution :
The body centered cubic cell is a type of closed packing where the atoms are present at every corner of the cube along with an atom at the body center.
The diagram below supports the above explanation of body centred cubic cell packing.
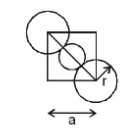
Based on the above diagram we can identify a triangle ${ \Delta DFB }$.
- In ${ \Delta DFB }$, we will apply Pythagoras theorem.
${ BF }^{ 2 }{ =DF }^{ 2 }{ +BD }^{ 2 }$
${ (\sqrt { 2a } ) }^{ 2 }{ +a }^{ 2 }$
${ 2a }^{ 2 }{ +a }^{ 2 }$
${ BF }^{ 2 }{= 3a }^{ 2 }$
${ BF=\sqrt { 3a } }$
${ \sqrt { 3a } =4r }$
${ a=4r/\sqrt { 3 } }$
Where r = radius of atom
a= edge length
- As we know that volume of a cube: ${ a }^{ 3 }$
= ${ (4r/\sqrt { 3 } )^{ 3 } }$
= ${ 64r }^{ 3 }{ /3\sqrt { 3 } }$
The volume of one sphere = ${ 4/3\pi r }^{ 3 }$
The number of particles present in body unit cell = ${ 8\times 1/8 + 1\times 1 }= { 1 + 1 } = { 2 }$
So, the total volume of sphere in a cube = ${ 4/3\pi r }^{ 3 }{ \times 2 }$
Use the formula,
Packing efficiency = ${ Volume\quad of\quad sphere/volume\quad of\quad cube\times 100 }$
= ${ 4/3\pi r }^{ 3 }{ \times 2\div 64r^{ 3 } }{ 3\sqrt { 3 } \times 100 }$
=\[68%\]
Therefore, we can conclude that the packing fraction of a body-centered cube is 68/100 i.e. 0.68.
So, the correct answer is “Option C”.
Additional Information:
- Atomic packing fraction is the volume of the atoms in a cell.
- The packing fraction of a nucleus is the ratio of its mass defects per no. of nucleons.
Packing fraction = mass defect/mass no.
- Packing fraction is directly related to its availability of nuclear energy and the stability of the nucleus.
- This fraction can have a positive or can have a negative sign. A positive packing fraction describes a tendency towards instability. A negative packing fraction means isotopic mass is less than the actual mass number.
Note: The possibility to make a mistake is that you must know the number of particles present in the body-centered unit cell and we have to take the total volume of the sphere.
Complete Solution :
The body centered cubic cell is a type of closed packing where the atoms are present at every corner of the cube along with an atom at the body center.
The diagram below supports the above explanation of body centred cubic cell packing.

Based on the above diagram we can identify a triangle ${ \Delta DFB }$.
- In ${ \Delta DFB }$, we will apply Pythagoras theorem.
${ BF }^{ 2 }{ =DF }^{ 2 }{ +BD }^{ 2 }$
${ (\sqrt { 2a } ) }^{ 2 }{ +a }^{ 2 }$
${ 2a }^{ 2 }{ +a }^{ 2 }$
${ BF }^{ 2 }{= 3a }^{ 2 }$
${ BF=\sqrt { 3a } }$
${ \sqrt { 3a } =4r }$
${ a=4r/\sqrt { 3 } }$
Where r = radius of atom
a= edge length
- As we know that volume of a cube: ${ a }^{ 3 }$
= ${ (4r/\sqrt { 3 } )^{ 3 } }$
= ${ 64r }^{ 3 }{ /3\sqrt { 3 } }$
The volume of one sphere = ${ 4/3\pi r }^{ 3 }$
The number of particles present in body unit cell = ${ 8\times 1/8 + 1\times 1 }= { 1 + 1 } = { 2 }$
So, the total volume of sphere in a cube = ${ 4/3\pi r }^{ 3 }{ \times 2 }$
Use the formula,
Packing efficiency = ${ Volume\quad of\quad sphere/volume\quad of\quad cube\times 100 }$
= ${ 4/3\pi r }^{ 3 }{ \times 2\div 64r^{ 3 } }{ 3\sqrt { 3 } \times 100 }$
=\[68%\]
Therefore, we can conclude that the packing fraction of a body-centered cube is 68/100 i.e. 0.68.
So, the correct answer is “Option C”.
Additional Information:
- Atomic packing fraction is the volume of the atoms in a cell.
- The packing fraction of a nucleus is the ratio of its mass defects per no. of nucleons.
Packing fraction = mass defect/mass no.
- Packing fraction is directly related to its availability of nuclear energy and the stability of the nucleus.
- This fraction can have a positive or can have a negative sign. A positive packing fraction describes a tendency towards instability. A negative packing fraction means isotopic mass is less than the actual mass number.
Note: The possibility to make a mistake is that you must know the number of particles present in the body-centered unit cell and we have to take the total volume of the sphere.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




