
The oxo acids of ${{P}_{2}}{{O}_{5}}$are ${{H}_{3}}P{{O}_{4,}}{{H}_{4}}{{P}_{2}}{{O}_{7,}}HP{{O}_{3,}}$ and ${{H}_{3}}P{{O}_{3}}$.
(A)-True
(B)-False
Answer
585.6k+ views
Hint: Phosphorus forms a number of oxoacids. The oxidation state of phosphorus in oxo acids is usually +1, +3,+4,+5. In ${{P}_{2}}{{O}_{5}}$, the oxidation state of phosphorus is+5. Composition of oxo acids depends on loss or gain of water molecules or oxygen atoms.
Complete step by step answer:
-In oxoacids phosphorus is surrounded by other atoms tetrahedrally.
-In ${{P}_{2}}{{O}_{5}}$, the oxidation state of phosphorus is +5. In oxo acids, oxygen atoms are attached to atoms.
-In oxo acids of ${{P}_{2}}{{O}_{5}}$ , phosphorus will have an oxidation state as +5 and will have phosphorus oxygen double bond.
-In ${{H}_{3}}P{{O}_{4}}$,
$\begin{align}
& {{H}_{3}}P{{O}_{4}} \\
& 3\times 1+P+4\times -2=0 \\
& 3+P+(-8)=0 \\
& P=+5 \\
\end{align}$
Oxidation state of phosphorus is +5. Three P-OH bonds and one P=O bond is present
The structure is as following:
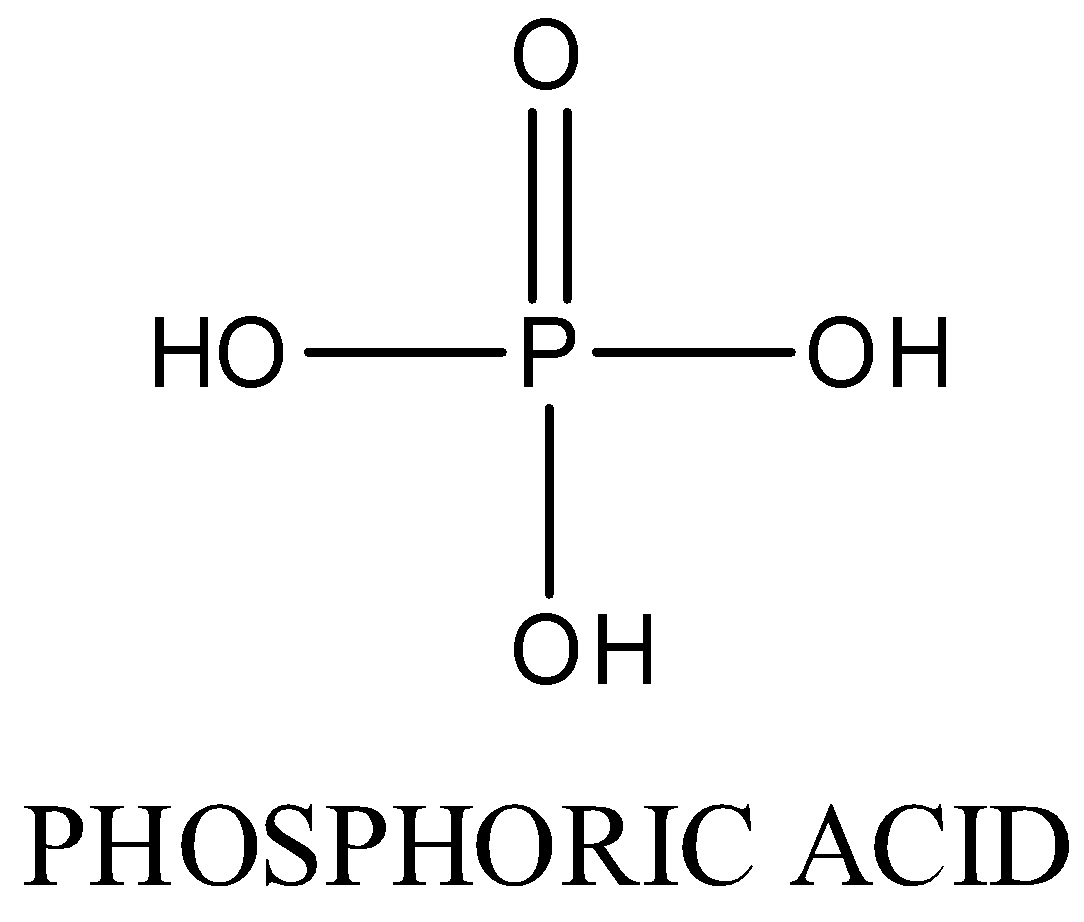
In ${{H}_{4}}{{P}_{2}}{{O}_{7}}$, Oxidation state of phosphorus is +5. Four P-OH bonds , two P=O bond and one P-O-P bond is present. The structure is as following:
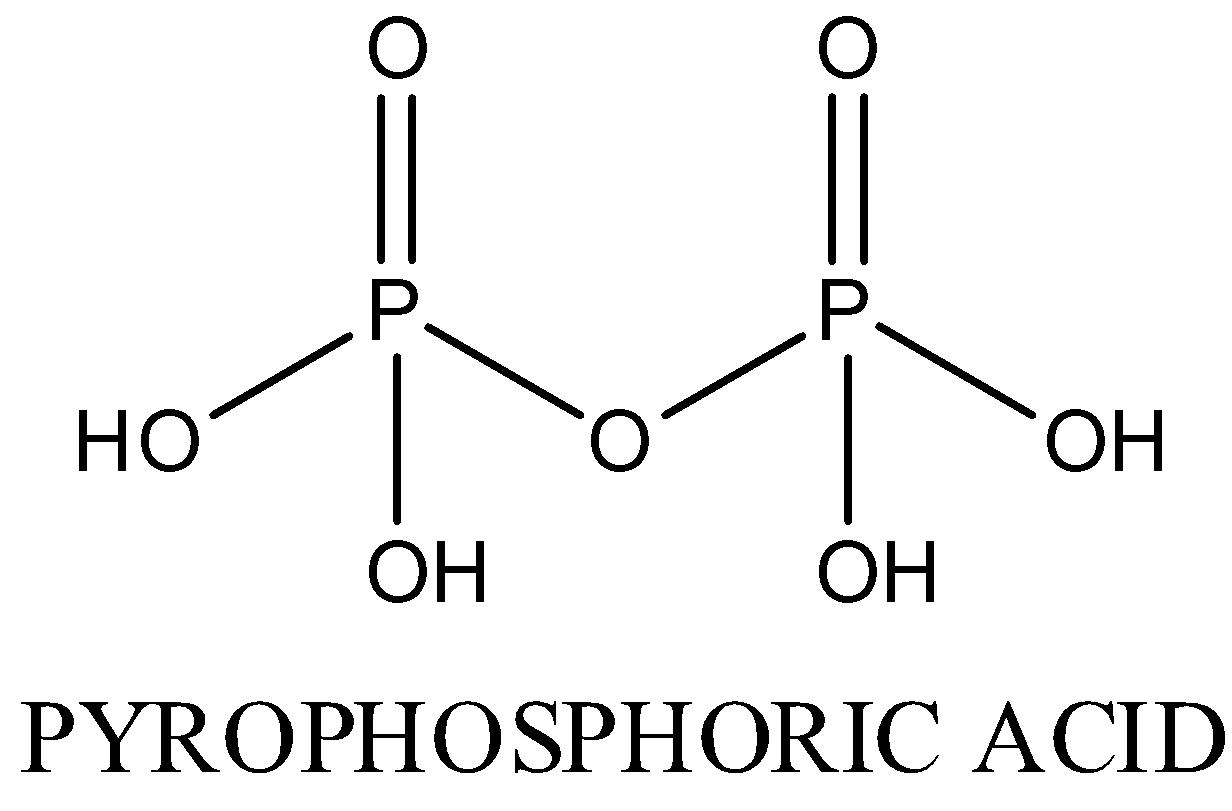
In $HP{{O}_{3}}$, oxidation state of phosphorous is +5.It exists in polymeric form.The structure is as following:
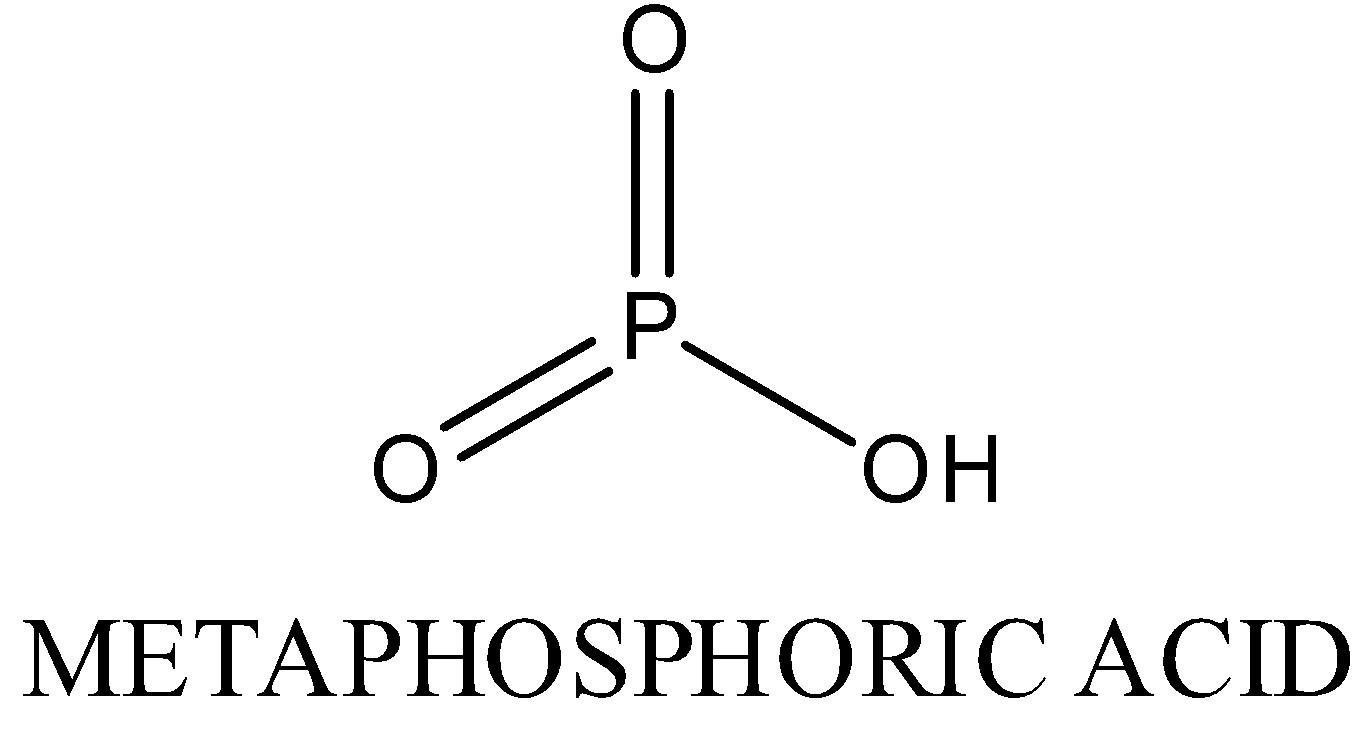
In ${{H}_{3}}P{{O}_{3}}$ , oxidation state of phosphorous is +3,Two P-OH bonds, one P=O bond, one P-H bond is present.
Structure is as following:
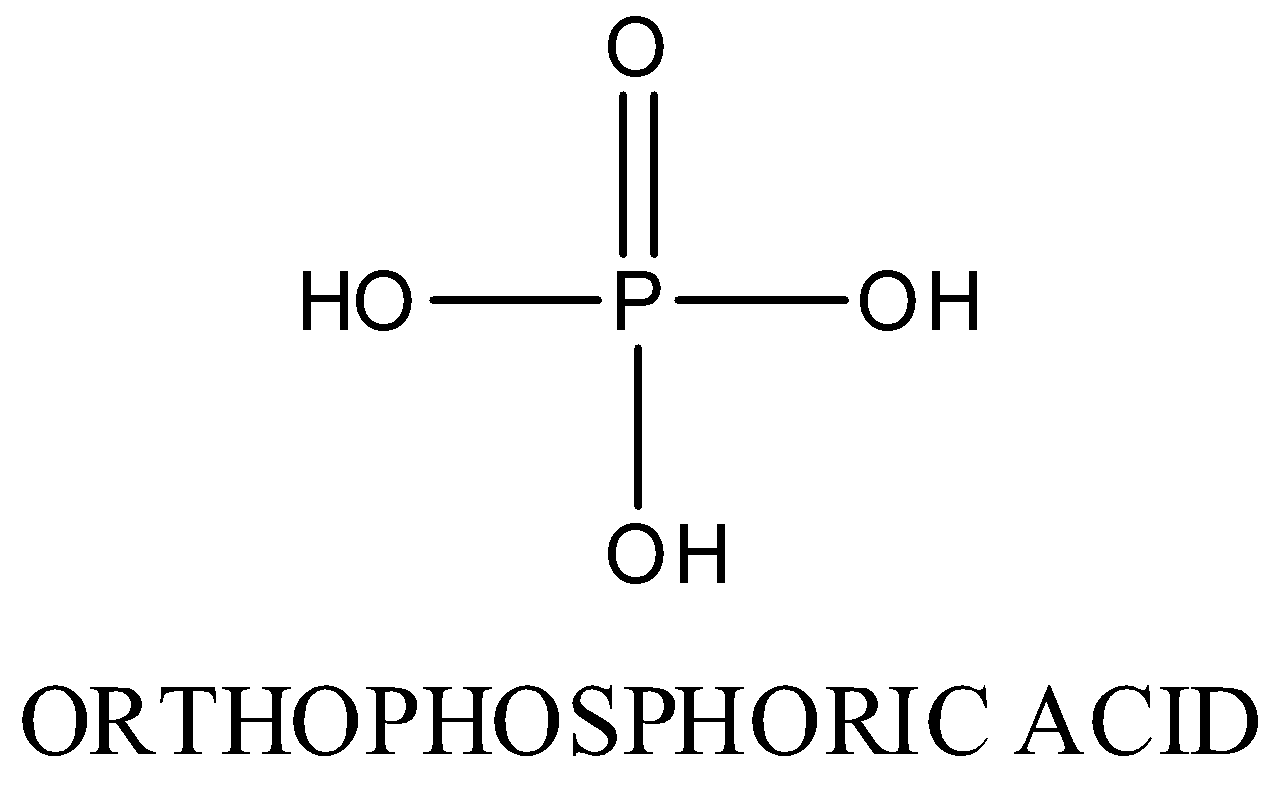
In ${{H}_{3}}P{{O}_{3}}$, oxidation number of phosphorus is +3, so it is not an oxo acid of ${{P}_{2}}{{O}_{5}}$.
Hence, statement The oxo acids of ${{P}_{2}}{{O}_{5}}$ are ${{H}_{3}}P{{O}_{4,}}{{H}_{4}}{{P}_{2}}{{O}_{7,}}HP{{O}_{3}}$ and ${{H}_{3}}P{{O}_{3}}$ is false.
So, the correct answer is “Option B”.
Note: Phosphorus pentoxide is produced by treating phosphorus with excess of oxygen. Phosphorus undergoes combustion to form phosphorus pentoxide.It is anhydride of orthophosphoric acid ${{H}_{3}}P{{O}_{3}}$ ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ is pyrophosphoric acid. $HP{{O}_{3}}$ is metaphosphoric acid. Oxoacid is an acid that contains oxygen atoms.
Complete step by step answer:
-In oxoacids phosphorus is surrounded by other atoms tetrahedrally.
-In ${{P}_{2}}{{O}_{5}}$, the oxidation state of phosphorus is +5. In oxo acids, oxygen atoms are attached to atoms.
-In oxo acids of ${{P}_{2}}{{O}_{5}}$ , phosphorus will have an oxidation state as +5 and will have phosphorus oxygen double bond.
-In ${{H}_{3}}P{{O}_{4}}$,
$\begin{align}
& {{H}_{3}}P{{O}_{4}} \\
& 3\times 1+P+4\times -2=0 \\
& 3+P+(-8)=0 \\
& P=+5 \\
\end{align}$
Oxidation state of phosphorus is +5. Three P-OH bonds and one P=O bond is present
The structure is as following:

In ${{H}_{4}}{{P}_{2}}{{O}_{7}}$, Oxidation state of phosphorus is +5. Four P-OH bonds , two P=O bond and one P-O-P bond is present. The structure is as following:

In $HP{{O}_{3}}$, oxidation state of phosphorous is +5.It exists in polymeric form.The structure is as following:

In ${{H}_{3}}P{{O}_{3}}$ , oxidation state of phosphorous is +3,Two P-OH bonds, one P=O bond, one P-H bond is present.
Structure is as following:

In ${{H}_{3}}P{{O}_{3}}$, oxidation number of phosphorus is +3, so it is not an oxo acid of ${{P}_{2}}{{O}_{5}}$.
Hence, statement The oxo acids of ${{P}_{2}}{{O}_{5}}$ are ${{H}_{3}}P{{O}_{4,}}{{H}_{4}}{{P}_{2}}{{O}_{7,}}HP{{O}_{3}}$ and ${{H}_{3}}P{{O}_{3}}$ is false.
So, the correct answer is “Option B”.
Note: Phosphorus pentoxide is produced by treating phosphorus with excess of oxygen. Phosphorus undergoes combustion to form phosphorus pentoxide.It is anhydride of orthophosphoric acid ${{H}_{3}}P{{O}_{3}}$ ${{H}_{4}}{{P}_{2}}{{O}_{7}}$ is pyrophosphoric acid. $HP{{O}_{3}}$ is metaphosphoric acid. Oxoacid is an acid that contains oxygen atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




