
The oxidation number of sulphur in Caro's acid (${{H}_{2}}S{{O}_{5}}$ ).
A. +5
B. +8
C. +6
D. +7
Answer
567k+ views
Hint: Caro's acid is also called Peroxymonosulfuric acid. At time of calculating the oxidation state we have to check whether the molecule contains a peroxide bond or not. If the molecule contains a peroxide bond then the oxidation state of the central atom is going to change.
Complete Solution :- In the question it is given that to calculate the oxidation number of sulphur in Caro's acid.
- To calculate the oxidation number of sulphur in Caro's acid we have to draw the structure of it and it is as follows.
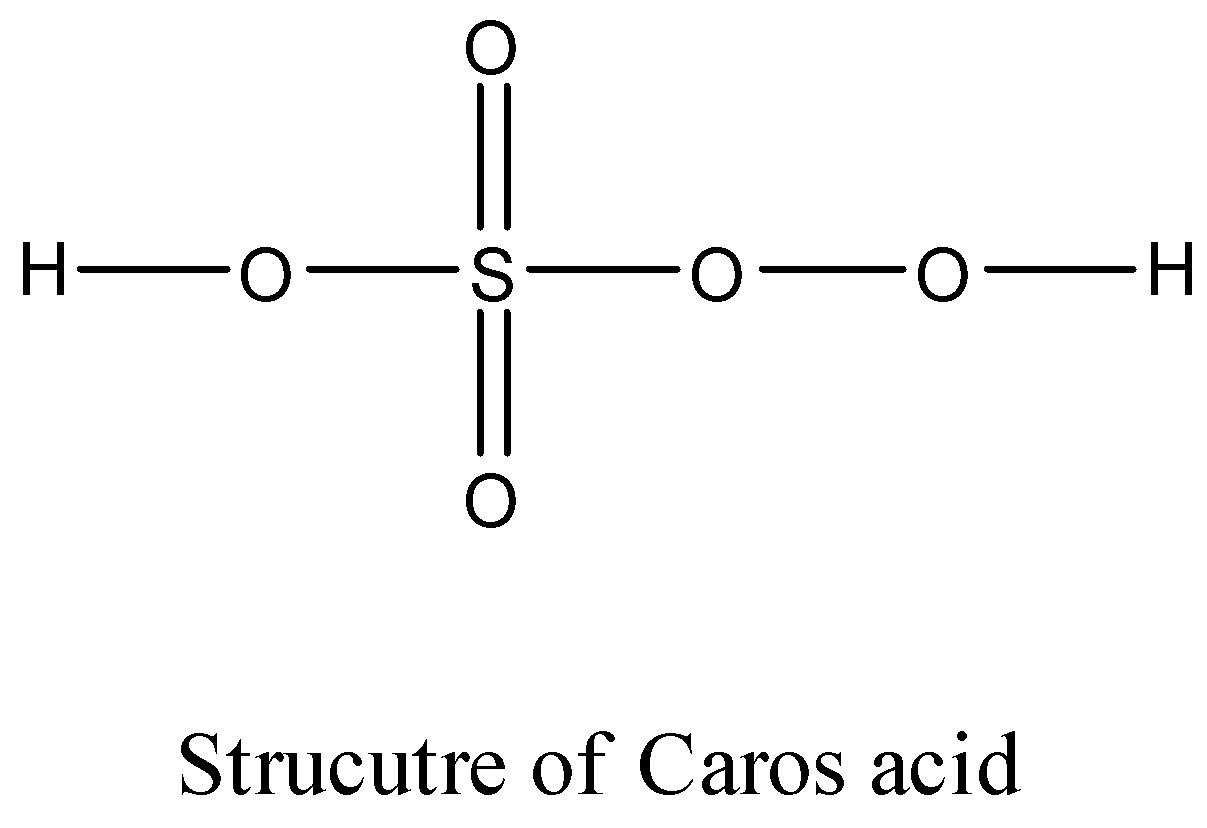
- We can write the oxidation state of each element in the given molecule and it is as follows.
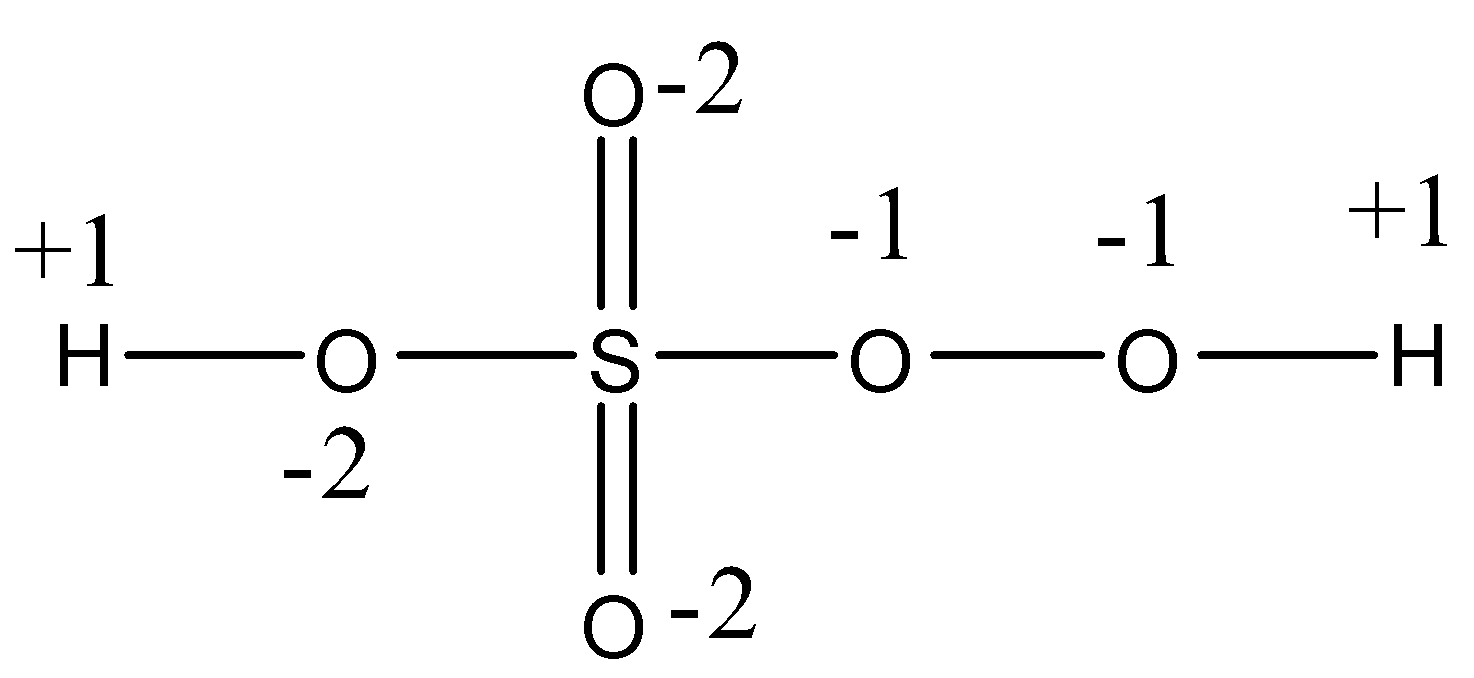
- Therefore the oxidation number of sulphur in Caro's acid is as follows.
x+ 3(O)+2(oxygen in peroxide bond)+2 (hydrogens) =0
x +3 (-2) +2 (-1) +2 (1) = 0
x - 6 -2 +2 = 0
x -6 = 0
x = 6.
Here x = oxidation state of sulphur
- Therefore the oxidation state of sulphur in Caro's acid is +6.
So, the correct answer is “Option C”.
Note: When we are going to calculate the oxidation number of an element in a peroxide molecule we are supposed to consider the oxidation number of oxygen in the peroxide bond is ‘-1’. If we consider the oxidation number of an oxygen in the peroxide bond as ‘-2’ then it will be wrong.
Complete Solution :- In the question it is given that to calculate the oxidation number of sulphur in Caro's acid.
- To calculate the oxidation number of sulphur in Caro's acid we have to draw the structure of it and it is as follows.

- We can write the oxidation state of each element in the given molecule and it is as follows.

- Therefore the oxidation number of sulphur in Caro's acid is as follows.
x+ 3(O)+2(oxygen in peroxide bond)+2 (hydrogens) =0
x +3 (-2) +2 (-1) +2 (1) = 0
x - 6 -2 +2 = 0
x -6 = 0
x = 6.
Here x = oxidation state of sulphur
- Therefore the oxidation state of sulphur in Caro's acid is +6.
So, the correct answer is “Option C”.
Note: When we are going to calculate the oxidation number of an element in a peroxide molecule we are supposed to consider the oxidation number of oxygen in the peroxide bond is ‘-1’. If we consider the oxidation number of an oxygen in the peroxide bond as ‘-2’ then it will be wrong.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




