
The oxidation number of $\text{ Cr }$ in $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$ is
A) $\text{ +10 }$
B) $\text{ +8 }$
C) $\text{ +6 }$
D) $\text{ +4 }$
Answer
584.7k+ views
Hint: The net charge on the neutral molecules is always equal to zero and the sum of the oxidation state of the elements present in the molecules or species is always equal to the charge. The$\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$is a butterfly structure. The $\text{ Cr }$ is bonded to the four oxygen from the peroxy linkage and the fifth oxygen forms a coordinate (normal) bond with the chromium.
Complete step by step answer:
The oxidation number is defined as the charge which is allocated to the atom or element in a chemical reaction. The charge on the atom may be due to the gain or loss of electrons. In short, the oxidation number is the charge on the atom which appears when it forms an ionic bond with the tom. It helps to describe the transfer of electrons from one element to another.
In molecules, the electronegative atoms gain an electron from other less electronegative species and thus its oxidation number decreases by the number of electrons acquired by it.
Let's consider the chromium pentoxide$\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$. The chromium has the electronic configuration of as follows:
$\text{ Cr = }\left[ \text{Ar} \right]\text{ 3}{{\text{d}}^{\text{4}}}\text{ 4}{{\text{s}}^{\text{2}}}\text{ }$ Or $\text{ Cr = }\left[ \text{Ar} \right]\text{ 3}{{\text{d}}^{5}}\text{ 4}{{\text{s}}^{1}}\text{ }$
There are five orbitals in the $\text{ 3d }$ orbitals and one electron in the $\text{ 4s }$ orbitals. This exception in the electronic configuration is observed because of the four oxygen in the$\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$. The chromium is bonded to the total of the five oxygen atoms.
The one oxygen is forming a coordinate bond with the chromium. This oxygen is in the normal state. The oxidation state of oxygen in the coordinate state is$\text{ }-\text{2 }$.
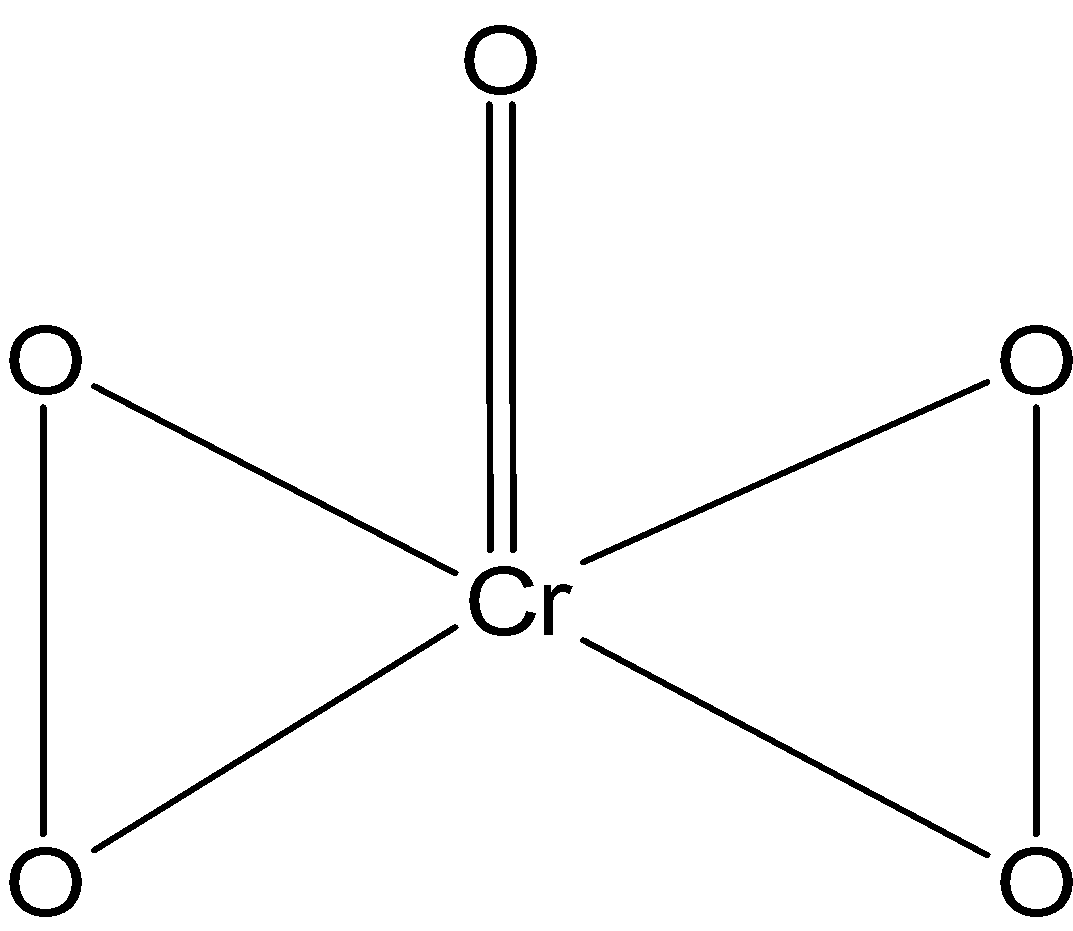
There are four oxygen which is bonded to the chromium atom. The oxygen in the peroxy linkage has the exceptional oxidation state of$\text{ }-1\text{ }$.
There are two peroxide linkages $\text{ }(-\text{O}-\text{O}-)\text{ }$ bonded to the chromium atom.
We are interested to find out the oxidation number of chromium in the$\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$. Let’s consider that the oxidation number of chromium as ‘X’.The $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$is a neutral molecule, therefore the total charge on the molecule is equals to the zero. We know that the oxidation state of the molecules is equal to the sum of the oxidation number of all elements present in the molecule is equal to the charge. Since then $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$has zero charges. We can say that the sum of the oxidation number of $\text{ Cr }$ and O will be equal to zero. It is as shown below,
$\text{ Charge on Cr}{{\text{O}}_{\text{5}}}\text{ = oxidation no}\text{. of Cr + Oxidation no}\text{. of O (Normal) + Oxidation no}\text{. of O (peroxide) }$
Let’s substitute the values for the oxidation number. We have,
$\begin{align}
& \text{ X + 4 (}-\text{1) }-\text{ 2 = 0} \\
& \Rightarrow \text{X }-4-2\text{ = 0 } \\
& \Rightarrow \text{ X = }+6 \\
\end{align}$
Therefore the oxidation number chromium in the $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$ is equal to $+6$.
Hence, (C) is the correct option.
Additional information:
There are certain guidelines to determine the oxidation number of the element. The oxidation number is either positive or negative.
1) The atoms which exist in its elemental form have zero oxidation number.
2) Elements which exist as a single atom or monoatomic have oxidation number equal to their charge.
3) In a species, the sum of the oxidation number of all the atoms is equal to the charge on the species.
4) Net charge on the neutral molecules is always equal to zero.
Note: Note that, at the first glance the oxidation state of the chromium may seem like the$\text{ +10 }$, but do not forget that the chromium cannot have the $\text{ +10 }$oxidation state. To know the oxidation state one should know the structure of the molecule. The $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$is a butterfly type structure and each oxygen in the peroxy linkage has an oxidation state of$\text{ }-1\text{ }$.
Complete step by step answer:
The oxidation number is defined as the charge which is allocated to the atom or element in a chemical reaction. The charge on the atom may be due to the gain or loss of electrons. In short, the oxidation number is the charge on the atom which appears when it forms an ionic bond with the tom. It helps to describe the transfer of electrons from one element to another.
In molecules, the electronegative atoms gain an electron from other less electronegative species and thus its oxidation number decreases by the number of electrons acquired by it.
Let's consider the chromium pentoxide$\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$. The chromium has the electronic configuration of as follows:
$\text{ Cr = }\left[ \text{Ar} \right]\text{ 3}{{\text{d}}^{\text{4}}}\text{ 4}{{\text{s}}^{\text{2}}}\text{ }$ Or $\text{ Cr = }\left[ \text{Ar} \right]\text{ 3}{{\text{d}}^{5}}\text{ 4}{{\text{s}}^{1}}\text{ }$
There are five orbitals in the $\text{ 3d }$ orbitals and one electron in the $\text{ 4s }$ orbitals. This exception in the electronic configuration is observed because of the four oxygen in the$\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$. The chromium is bonded to the total of the five oxygen atoms.
The one oxygen is forming a coordinate bond with the chromium. This oxygen is in the normal state. The oxidation state of oxygen in the coordinate state is$\text{ }-\text{2 }$.

There are four oxygen which is bonded to the chromium atom. The oxygen in the peroxy linkage has the exceptional oxidation state of$\text{ }-1\text{ }$.
There are two peroxide linkages $\text{ }(-\text{O}-\text{O}-)\text{ }$ bonded to the chromium atom.
We are interested to find out the oxidation number of chromium in the$\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$. Let’s consider that the oxidation number of chromium as ‘X’.The $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$is a neutral molecule, therefore the total charge on the molecule is equals to the zero. We know that the oxidation state of the molecules is equal to the sum of the oxidation number of all elements present in the molecule is equal to the charge. Since then $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$has zero charges. We can say that the sum of the oxidation number of $\text{ Cr }$ and O will be equal to zero. It is as shown below,
$\text{ Charge on Cr}{{\text{O}}_{\text{5}}}\text{ = oxidation no}\text{. of Cr + Oxidation no}\text{. of O (Normal) + Oxidation no}\text{. of O (peroxide) }$
Let’s substitute the values for the oxidation number. We have,
$\begin{align}
& \text{ X + 4 (}-\text{1) }-\text{ 2 = 0} \\
& \Rightarrow \text{X }-4-2\text{ = 0 } \\
& \Rightarrow \text{ X = }+6 \\
\end{align}$
Therefore the oxidation number chromium in the $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$ is equal to $+6$.
Hence, (C) is the correct option.
Additional information:
There are certain guidelines to determine the oxidation number of the element. The oxidation number is either positive or negative.
1) The atoms which exist in its elemental form have zero oxidation number.
2) Elements which exist as a single atom or monoatomic have oxidation number equal to their charge.
3) In a species, the sum of the oxidation number of all the atoms is equal to the charge on the species.
4) Net charge on the neutral molecules is always equal to zero.
Note: Note that, at the first glance the oxidation state of the chromium may seem like the$\text{ +10 }$, but do not forget that the chromium cannot have the $\text{ +10 }$oxidation state. To know the oxidation state one should know the structure of the molecule. The $\text{ Cr}{{\text{O}}_{\text{5}}}\text{ }$is a butterfly type structure and each oxygen in the peroxy linkage has an oxidation state of$\text{ }-1\text{ }$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




