
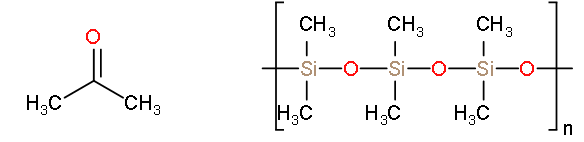
The organic solvent acetone has the molecular formula \[{{(\text{C}{{\text{H}}_{3}}\text{)}}_{2}}\text{CO}\]. The silicon analogue, a thermally stable lubricant, is a polymer, \[{{\left( {{(\text{C}{{\text{H}}_{3}}\text{)}}_{2}}\text{SiO} \right)}_{\text{n}}}\]. Account for the difference in structure.

Answer
588.9k+ views
Hint: Those elements which are present in the similar chemical properties as the outermost valence electron is the same in all the elements of the same group. Also, elements of the same group have similar physical properties and have the same bonding but the position of an atom is different.
Complete step by step Answer:
-In the given question, we have to tell why there is a difference in the structure of the acetone and silicon.
-As we know that elements which are present in the same group have the same number of valence electrons due to which they have the same chemical and physical properties.
-The carbon and silicon both are present in group 14 and have electronic configuration of \[\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{2}}\text{ and 1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{6}}\text{ 3}{{\text{s}}^{2}}\text{ 3}{{\text{p}}^{2}}\]
-Now, due to the presence of the same valence electrons, both carbon and silicon have the same ability to make 4 bond pairs.
-In acetone, carbon makes two bonds with oxygen and two bonds with the carbon similarly silicon makes two bonds with oxygen and two bonds with carbon.
-But when the silicon structure undergoes a polymerization reaction it forms polymethyl siloxane.
-So, according to the given structures the acetone is the single unit but the silicon forms a long chain by combining about 100 units.
-That's why there is a difference in the structure of both elements.
Note: Poly Methyl siloxane is a polymer which is made by the repetition of the monomer dimethyldichlorosilane. It is also known as an anti-foaming agent. PDMS or Poly Methyl siloxane is used as an emulsifier in the food.
Complete step by step Answer:
-In the given question, we have to tell why there is a difference in the structure of the acetone and silicon.
-As we know that elements which are present in the same group have the same number of valence electrons due to which they have the same chemical and physical properties.
-The carbon and silicon both are present in group 14 and have electronic configuration of \[\text{1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{2}}\text{ and 1}{{\text{s}}^{2}}\text{ 2}{{\text{s}}^{2}}\text{ 2}{{\text{p}}^{6}}\text{ 3}{{\text{s}}^{2}}\text{ 3}{{\text{p}}^{2}}\]
-Now, due to the presence of the same valence electrons, both carbon and silicon have the same ability to make 4 bond pairs.
-In acetone, carbon makes two bonds with oxygen and two bonds with the carbon similarly silicon makes two bonds with oxygen and two bonds with carbon.
-But when the silicon structure undergoes a polymerization reaction it forms polymethyl siloxane.
-So, according to the given structures the acetone is the single unit but the silicon forms a long chain by combining about 100 units.
-That's why there is a difference in the structure of both elements.
Note: Poly Methyl siloxane is a polymer which is made by the repetition of the monomer dimethyldichlorosilane. It is also known as an anti-foaming agent. PDMS or Poly Methyl siloxane is used as an emulsifier in the food.
Recently Updated Pages
Master Class 12 Business Studies: Engaging Questions & Answers for Success

Master Class 12 Biology: Engaging Questions & Answers for Success

Master Class 12 Chemistry: Engaging Questions & Answers for Success

Class 12 Question and Answer - Your Ultimate Solutions Guide

Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE




