
The order of decreasing reactivity towards the electrophilic substitution is:
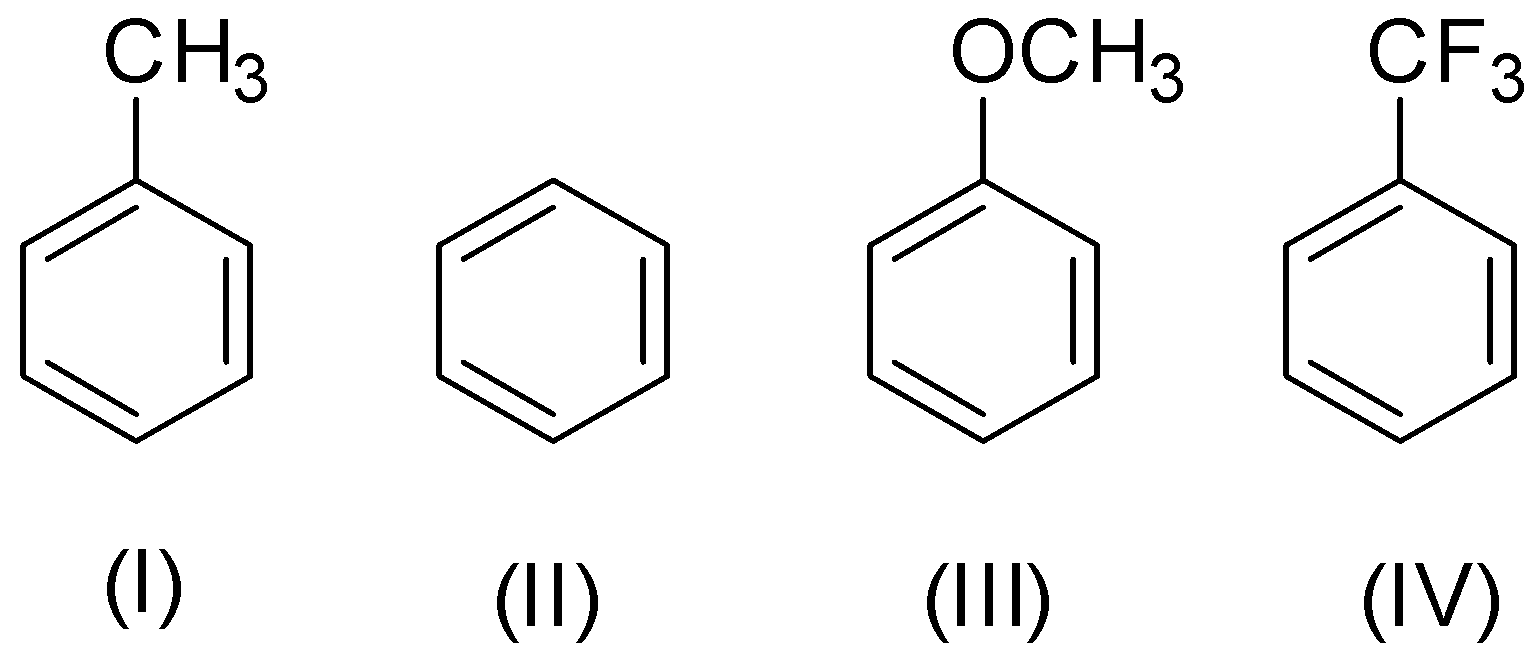
A.II>I>III>IV
B.III>I>II>IV
C.IV>I>II>III
D.I>II>III>IV

Answer
591.6k+ views
Hint: A substitution reaction in which the functional group present in a molecule or attached to the molecule is substituted or replaced by an electrophile is termed as ‘electrophilic substitution reaction’.
The two main types of electrophilic substitution reactions undergone by organic compounds are electrophilic aromatic substitution reactions and electrophilic aliphatic substitution reactions.
In electrophilic aromatic substitution reactions, an atom attached to an aromatic ring is substituted by an electrophile whereas in electrophilic aliphatic substitution reactions, an electrophile replaces the functional group of an aliphatic compound.
Complete step by step answer:
In chemistry, the term ‘electron withdrawing group’ refers to the type of atoms or groups which can attract or pull the electron density of the atoms in their neighborhood towards themselves by the inductive effect or resonance effect. Examples of electron withdrawing groups are ${\text{ - F, - Cl, - C}}{{\text{F}}_{\text{3}}}{\text{, - CN, - OCOR}}$ etc.
On the other hand, the term ‘electron donating group’ or ‘electron releasing group’ refers to the type of atoms or groups which can donate or release their electron densities to the atoms in their neighborhood. Examples of electron donating groups are ${\text{ - R, - N}}{{\text{H}}_{\text{2}}}{\text{, - N}}{{\text{R}}_{\text{2}}}$ etc.
In electrophilic aromatic substitution reaction, benzene acts as a nucleophile that means it needs to donate electrons from inside its ring. So, when deactivating groups are present on the benzene ring, benzene becomes less reactive towards electrophilic aromatic substitution and when activating groups are present, it becomes more reactive towards electrophilic aromatic substitution.
Now, generally electron withdrawing groups are deactivating groups and electron donating groups are activating groups.
Now, in (IV), an electron withdrawing group ${\text{ - C}}{{\text{F}}_{\text{3}}}$ is present and so the ring is less reactive towards electrophilic aromatic substitution.
In (I) and (III), electron donating methyl group and methoxy group are present respectively. So the ring becomes more reactive towards electrophilic aromatic substitution but the effect of methoxy group is more than methyl group.
So, the decreasing order of reactivity is III>I>II>IV which is given by option B.
Hence option B is correct.
Note:
Electrophilic substitution reactions usually proceed through a 3 step mechanism. The 3 steps involved in this mechanism are the generation of an electrophile followed by the formation of a carbocation intermediate and finally the removal of a proton from the intermediate.
The two main types of electrophilic substitution reactions undergone by organic compounds are electrophilic aromatic substitution reactions and electrophilic aliphatic substitution reactions.
In electrophilic aromatic substitution reactions, an atom attached to an aromatic ring is substituted by an electrophile whereas in electrophilic aliphatic substitution reactions, an electrophile replaces the functional group of an aliphatic compound.
Complete step by step answer:
In chemistry, the term ‘electron withdrawing group’ refers to the type of atoms or groups which can attract or pull the electron density of the atoms in their neighborhood towards themselves by the inductive effect or resonance effect. Examples of electron withdrawing groups are ${\text{ - F, - Cl, - C}}{{\text{F}}_{\text{3}}}{\text{, - CN, - OCOR}}$ etc.
On the other hand, the term ‘electron donating group’ or ‘electron releasing group’ refers to the type of atoms or groups which can donate or release their electron densities to the atoms in their neighborhood. Examples of electron donating groups are ${\text{ - R, - N}}{{\text{H}}_{\text{2}}}{\text{, - N}}{{\text{R}}_{\text{2}}}$ etc.
In electrophilic aromatic substitution reaction, benzene acts as a nucleophile that means it needs to donate electrons from inside its ring. So, when deactivating groups are present on the benzene ring, benzene becomes less reactive towards electrophilic aromatic substitution and when activating groups are present, it becomes more reactive towards electrophilic aromatic substitution.
Now, generally electron withdrawing groups are deactivating groups and electron donating groups are activating groups.
Now, in (IV), an electron withdrawing group ${\text{ - C}}{{\text{F}}_{\text{3}}}$ is present and so the ring is less reactive towards electrophilic aromatic substitution.
In (I) and (III), electron donating methyl group and methoxy group are present respectively. So the ring becomes more reactive towards electrophilic aromatic substitution but the effect of methoxy group is more than methyl group.
So, the decreasing order of reactivity is III>I>II>IV which is given by option B.
Hence option B is correct.
Note:
Electrophilic substitution reactions usually proceed through a 3 step mechanism. The 3 steps involved in this mechanism are the generation of an electrophile followed by the formation of a carbocation intermediate and finally the removal of a proton from the intermediate.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




