
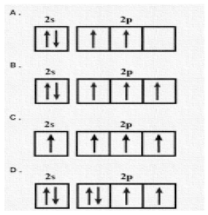
The orbital diagram in which the Aufbau principle is violated is:

Answer
582k+ views
Hint: The Aufbau principle is known to us as it dictates the manner in which electrons are filled in the atomic orbitals of an atom in its ground state. It clearly states that electrons are filled into atomic orbitals in the increasing order of orbital energy level i.e. an electron will occupy an orbital of lower energy level followed by the higher energy levels.
Complete step-by-step answer:
According to the Aufbau principle, from the available atomic orbitals, the one which has the lowest energy level is occupied before those with higher energy levels. It is important to note that each orbital can hold a maximum of two electrons as stated by the Pauli exclusion principle.
The word ‘Aufbau’ is a German word and can be translated as construct or build up. The Aufbau principle can be used to understand the position of electrons in an atom and their corresponding energy levels. The manner in which electrons are filled into orbitals in a single subshell should follow Hund’s rule which says that every orbital in a given subshell must be singly occupied by electrons before any two electrons pair up in an orbital.
There are some important features which we must focus on while knowing about Aufbau principle. These are:
1.According to this principle, electrons first occupy those orbitals whose energy is the lowest such that the electrons enter the orbitals having higher energies only when orbitals with lower energies are completely filled.
2.We can determine the order in which the energy of orbitals increases with the help of the (n+l) rule, where the sum of the principal (n) and azimuthal quantum numbers (l) tells us the energy level of the orbital.
3.Lower (n+l) values correspond to lower orbital energies. But in case two orbitals share equal (n+l) values, the orbital having lower n value is said to have lower energy associated with it.
4.The order we follow to fill the orbital with electrons is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
Now, if we look at the options, we can see that we are provided with 2s and 2p orbitals. So, as per Aufbau principle, first electrons are filled in 1s orbital. Since s electrons can occupy a maximum of two electrons, once it is filled with two electrons, the third one enters in 2s orbital as per sequence of increasing energy levels. It can also accommodate two electrons.
Once it is completely filled by two electrons, the next energy level is 2p orbital which can accommodate 6 electrons. We know that \[2{p_x},2{p_y},2{p_z}\] are the degenerate orbitals and thus have the same energy levels. So, the electrons can occupy either of three in any order. In this way, filing of orbitals goes on as per Aufbau principle.
No, looking at the option give, we can easily find out that Aufbau principle is violated in the option C as 2s is not completely filled, yet electrons are filled in 2p orbital which has higher energy than 2s. and we already know that 2s must be completely filled by two electrons followed by the 2p orbital as per Aufbau rule.
Hence, the correct option is (C).
Note:The electron configuration of chromium is an exception due to several factors such as the increased stability provided by half-filled subshells and the relatively low energy gap between the 3d and the 4s subshells. Due to this 3d orbital is completely filled first followed by 4s. Similarly, in copper due to the stability provided by a completely filled 3d subshell.
Complete step-by-step answer:
According to the Aufbau principle, from the available atomic orbitals, the one which has the lowest energy level is occupied before those with higher energy levels. It is important to note that each orbital can hold a maximum of two electrons as stated by the Pauli exclusion principle.
The word ‘Aufbau’ is a German word and can be translated as construct or build up. The Aufbau principle can be used to understand the position of electrons in an atom and their corresponding energy levels. The manner in which electrons are filled into orbitals in a single subshell should follow Hund’s rule which says that every orbital in a given subshell must be singly occupied by electrons before any two electrons pair up in an orbital.
There are some important features which we must focus on while knowing about Aufbau principle. These are:
1.According to this principle, electrons first occupy those orbitals whose energy is the lowest such that the electrons enter the orbitals having higher energies only when orbitals with lower energies are completely filled.
2.We can determine the order in which the energy of orbitals increases with the help of the (n+l) rule, where the sum of the principal (n) and azimuthal quantum numbers (l) tells us the energy level of the orbital.
3.Lower (n+l) values correspond to lower orbital energies. But in case two orbitals share equal (n+l) values, the orbital having lower n value is said to have lower energy associated with it.
4.The order we follow to fill the orbital with electrons is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.

Now, if we look at the options, we can see that we are provided with 2s and 2p orbitals. So, as per Aufbau principle, first electrons are filled in 1s orbital. Since s electrons can occupy a maximum of two electrons, once it is filled with two electrons, the third one enters in 2s orbital as per sequence of increasing energy levels. It can also accommodate two electrons.
Once it is completely filled by two electrons, the next energy level is 2p orbital which can accommodate 6 electrons. We know that \[2{p_x},2{p_y},2{p_z}\] are the degenerate orbitals and thus have the same energy levels. So, the electrons can occupy either of three in any order. In this way, filing of orbitals goes on as per Aufbau principle.
No, looking at the option give, we can easily find out that Aufbau principle is violated in the option C as 2s is not completely filled, yet electrons are filled in 2p orbital which has higher energy than 2s. and we already know that 2s must be completely filled by two electrons followed by the 2p orbital as per Aufbau rule.
Hence, the correct option is (C).
Note:The electron configuration of chromium is an exception due to several factors such as the increased stability provided by half-filled subshells and the relatively low energy gap between the 3d and the 4s subshells. Due to this 3d orbital is completely filled first followed by 4s. Similarly, in copper due to the stability provided by a completely filled 3d subshell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




