
The orbital diagram in which Hund’s rule is violated is:
A.
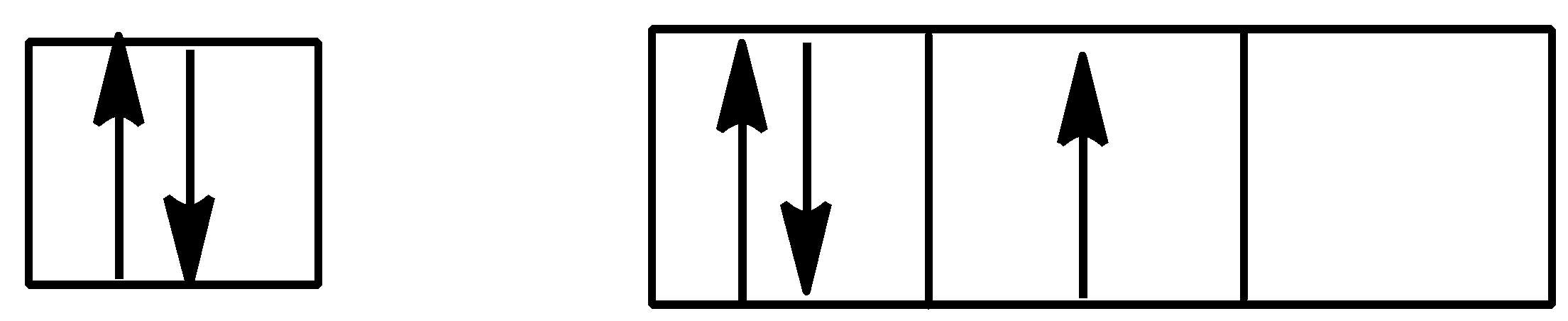
B.
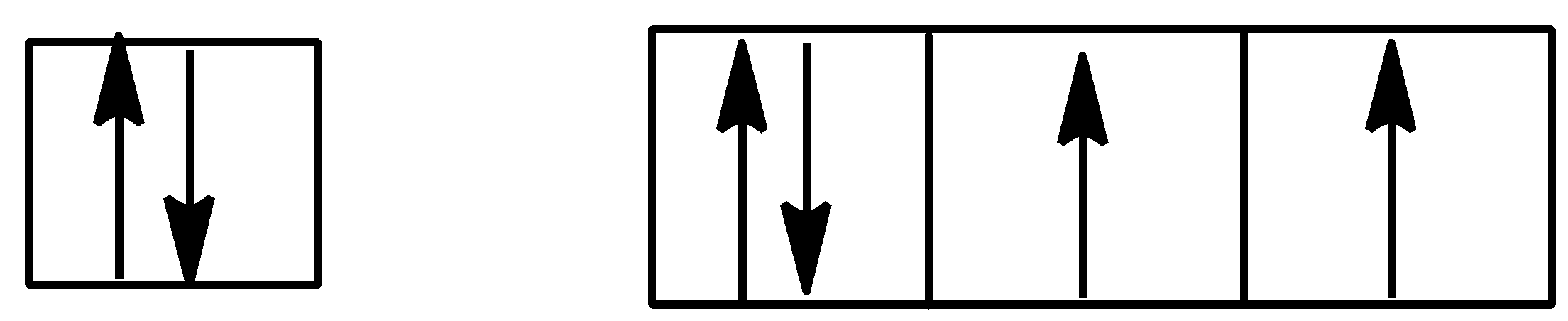
C.
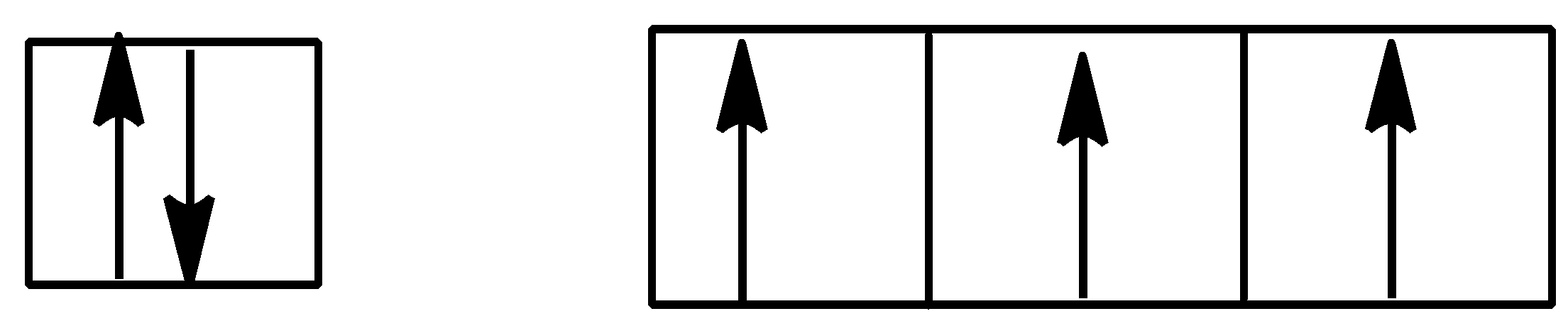
D.
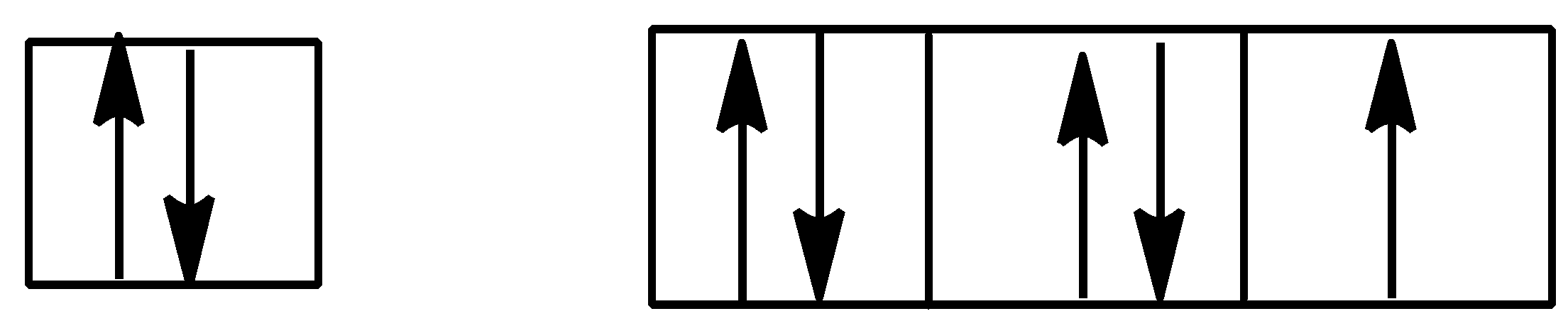




Answer
579.9k+ views
Hint: We know that the distribution of electrons in different orbitals is termed as electronic configuration. Three rules to be followed while deciding electronic configuration of an atom, they are, Hund’s rule, Pauli’s exclusion principle and Aufbau’s principle.
Complete step by step answer:
Let’s discuss Hund’s rule in detail. Hund’s rule states that each subshell in an orbital must be filled with one electron each before anyone is doubly occupied and the spin of all electrons in singly occupied shells is the same.
Let’s identify the correct answer from the options.
Option A is,
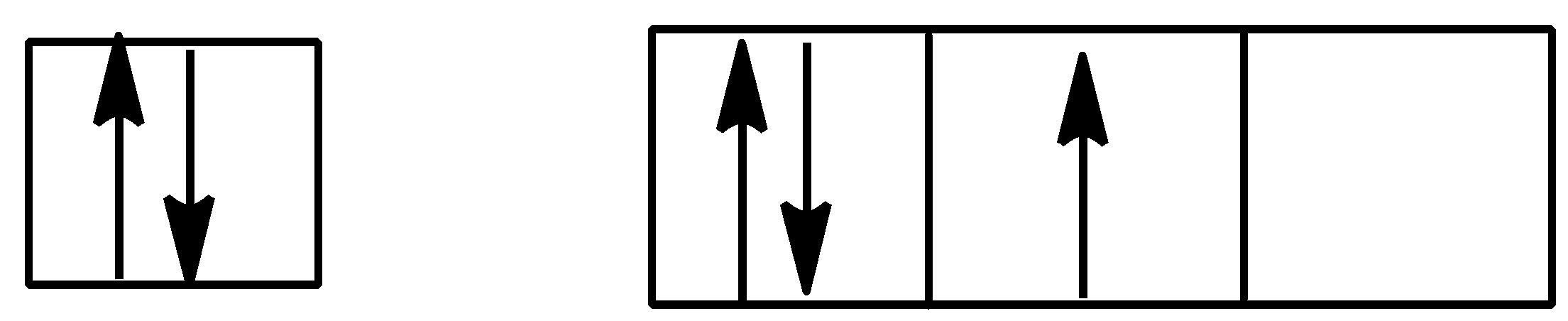
Here, in p orbital one subshell is vacant but one subshell is doubly filled or full filled. This type of electronic configuration violated the Hund’s rule.
Option B is,
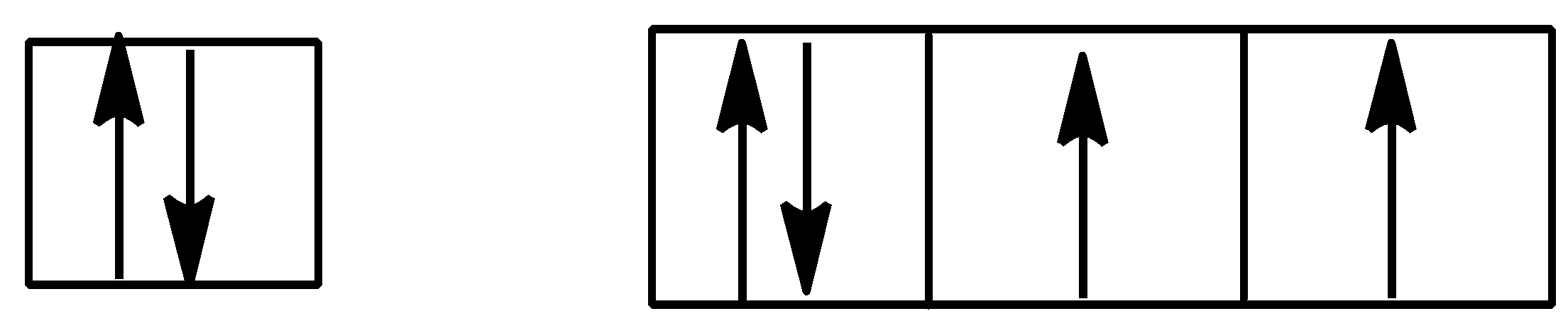
Here, Hund’s rule is followed as all subshells are singly filled before getting doubly filled.
Option C is,
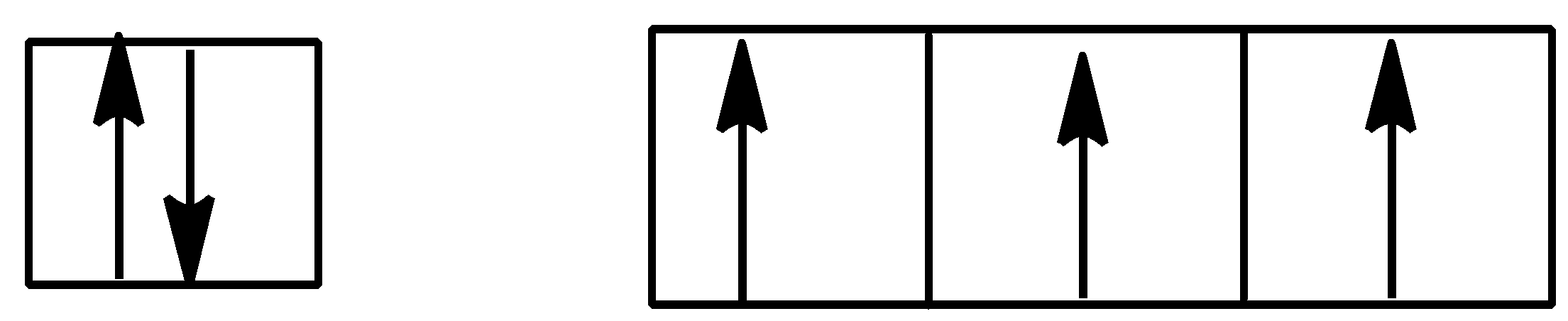
Here also Hund’s rule is followed as all subshells are singly filled.
Option D is,
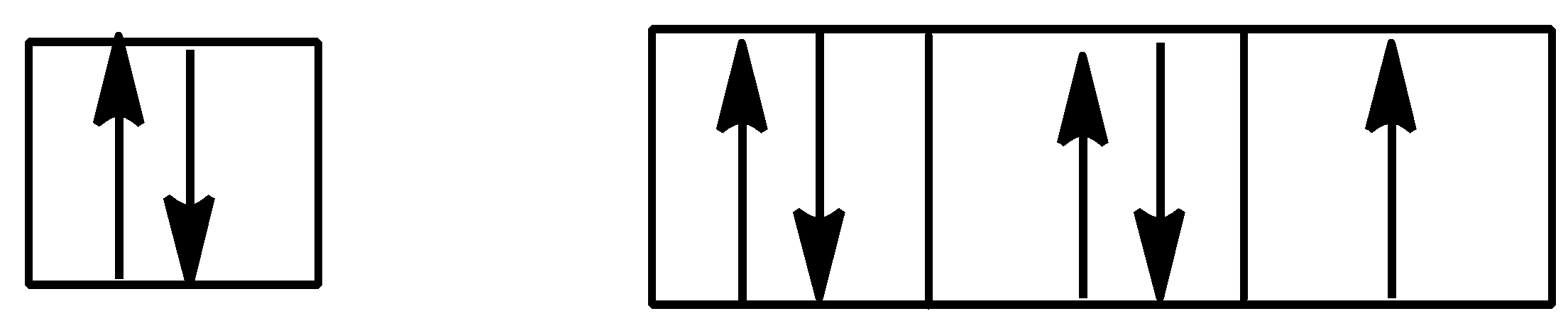
Here also, Hund’s rule is followed as all subshells are singly filled first before getting doubly filled.
Therefore, in option A Hund’s rule is violated. Hence, the correct answer is option A.
Additional Information
According to Pauli’s exclusion principle, all subshells in an orbital can accommodate a maximum of two electrons and spin of both the electrons is opposite to each other.
Note:
Aufbau principle also must be followed while deciding electronic configuration of an atom. According to this principle, electrons should be filled in lower energy orbitals first and then should be filled in higher energy orbitals.
Complete step by step answer:
Let’s discuss Hund’s rule in detail. Hund’s rule states that each subshell in an orbital must be filled with one electron each before anyone is doubly occupied and the spin of all electrons in singly occupied shells is the same.
Let’s identify the correct answer from the options.
Option A is,

Here, in p orbital one subshell is vacant but one subshell is doubly filled or full filled. This type of electronic configuration violated the Hund’s rule.
Option B is,

Here, Hund’s rule is followed as all subshells are singly filled before getting doubly filled.
Option C is,

Here also Hund’s rule is followed as all subshells are singly filled.
Option D is,

Here also, Hund’s rule is followed as all subshells are singly filled first before getting doubly filled.
Therefore, in option A Hund’s rule is violated. Hence, the correct answer is option A.
Additional Information
According to Pauli’s exclusion principle, all subshells in an orbital can accommodate a maximum of two electrons and spin of both the electrons is opposite to each other.
Note:
Aufbau principle also must be followed while deciding electronic configuration of an atom. According to this principle, electrons should be filled in lower energy orbitals first and then should be filled in higher energy orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




