
The number of unpaired electrons present in Fe+3 is :
A. 5
B. 4
C. 3
D. 6
Answer
585.3k+ views
Hint: According to the Bohr's model in the atoms the electron is moving around the nucleus in different shells. These shells are K, L, M, N, etc. Electronic distribution of an atom/ion is an arrangement in a space of the electrons around the center of the mass(nucleus) of the atom or ion among the orbits or shells.
Complete step by step answer:
All elements have a characteristic property known as the atomic number. The atomic number of an element represents the number of electrons or protons present in the atom of the element. On the other hand, Aufbau’s Principle helps in determining the order in which the electron orbitals get filled. Using both these concepts, we can determine the electronic configuration of the given element.
Iron (Fe) is a d- block metal. The d block elements are found in the group \[3,4,5,6,7,8,9,10,11\] and $12$ of the periodic table. These are also known as transition metals. The d orbital is filled with an electronic shell $n - 1$. There is a total of 40 d block elements.
D orbitals have 5 subshells and each subshell is capable of carrying 2 electrons. This means that the total capacity of the d orbital is 10 electrons. we need to understand how the unpaired d-orbital electrons in these transition elements d-orbital. There are five orbitals present in the d subshell. As the number of unpaired valence electrons increases, the d-orbital increases, the highest oxidation state increases.
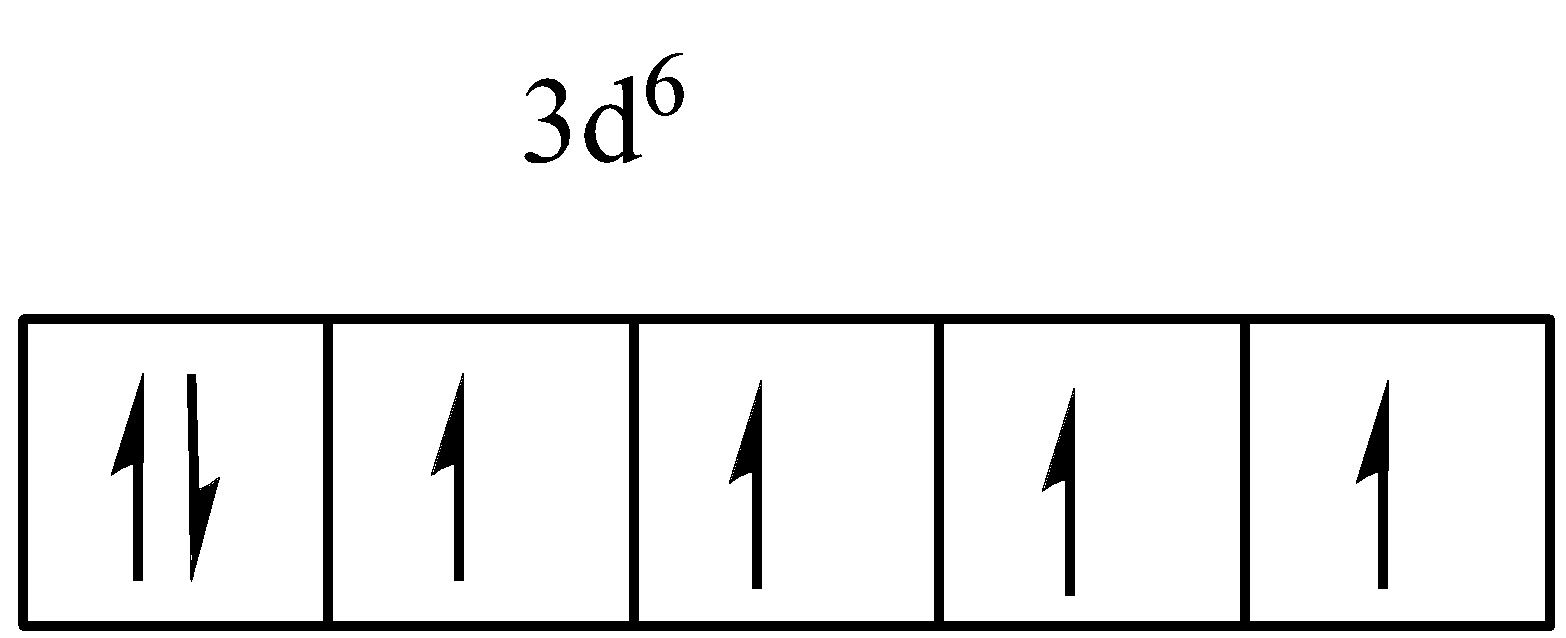
The atomic number of Fe is 26. The electronic configuration is, \[[Ar]4{s^2}3{d^6}\] . Now \[F{e^{ + 2}}\] has removed two electrons from its 4s orbital. And the electronic configuration becomes, \[[Ar]3{d^6}4{s^0}\] . From this electronic configuration, it is clear that \[F{e^{ + 2}}\] has 4 unpaired electrons in its d orbital. The diagram is shown below.
So, the correct answer is B.
Note:Now, to determine the period in which the element is placed, we need to look at the principal quantum number of the valence electron.
Let us solve an example to understand this concept. Let us consider Iron:
1.The atomic number of irons is 26. Hence the electronic configuration is given as \[[Ar]3{d^6}4{s^2}\] .
2.Since the valence electron enters into 3d subshell, the given element belongs to the d block.
3.The principal quantum number of the valence electron of Fe is 3. Hence, it belongs to the 3rd period.
4.Since iron belongs to d block, its group number can be calculated by using:
[number of electrons in (n-1) d subshell] + (number of electrons in (n) s subshell)
= (6) + (2) = 8
Hence, iron belongs to the 8th group.
Complete step by step answer:
All elements have a characteristic property known as the atomic number. The atomic number of an element represents the number of electrons or protons present in the atom of the element. On the other hand, Aufbau’s Principle helps in determining the order in which the electron orbitals get filled. Using both these concepts, we can determine the electronic configuration of the given element.
Iron (Fe) is a d- block metal. The d block elements are found in the group \[3,4,5,6,7,8,9,10,11\] and $12$ of the periodic table. These are also known as transition metals. The d orbital is filled with an electronic shell $n - 1$. There is a total of 40 d block elements.
D orbitals have 5 subshells and each subshell is capable of carrying 2 electrons. This means that the total capacity of the d orbital is 10 electrons. we need to understand how the unpaired d-orbital electrons in these transition elements d-orbital. There are five orbitals present in the d subshell. As the number of unpaired valence electrons increases, the d-orbital increases, the highest oxidation state increases.
The atomic number of Fe is 26. The electronic configuration is, \[[Ar]4{s^2}3{d^6}\] . Now \[F{e^{ + 2}}\] has removed two electrons from its 4s orbital. And the electronic configuration becomes, \[[Ar]3{d^6}4{s^0}\] . From this electronic configuration, it is clear that \[F{e^{ + 2}}\] has 4 unpaired electrons in its d orbital. The diagram is shown below.

So, the correct answer is B.
Note:Now, to determine the period in which the element is placed, we need to look at the principal quantum number of the valence electron.
Let us solve an example to understand this concept. Let us consider Iron:
1.The atomic number of irons is 26. Hence the electronic configuration is given as \[[Ar]3{d^6}4{s^2}\] .
2.Since the valence electron enters into 3d subshell, the given element belongs to the d block.
3.The principal quantum number of the valence electron of Fe is 3. Hence, it belongs to the 3rd period.
4.Since iron belongs to d block, its group number can be calculated by using:
[number of electrons in (n-1) d subshell] + (number of electrons in (n) s subshell)
= (6) + (2) = 8
Hence, iron belongs to the 8th group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




