
The number of structural and configurational isomers of a bromo compound
\[{{C}_{5}}{{H}_{9}}Br\], formed by the addition of HBr to 2-pentyne respectively are:
(a)- 1 and 2
(b)- 2 and 4
(c)- 4 and 2
(d)- 2 and 1
Answer
600k+ views
Hint: Isomers are the molecules which have the same molecular formula, but have a different arrangement of the atoms. They have the same empirical formula but they do not necessarily share similar properties.
Complete step by step solution:
Hydrogen bromide and 2-pentyne react together to form 3-bromopent-2-yne and 2-bromopent-2-yne as a final product. The reaction is given as:
\[2C{{H}_{3}}-C\equiv C-{{C}_{2}}{{H}_{5}}+2HBr\to C{{H}_{3}}-(Br)C=CH-{{C}_{2}}{{H}_{5}}+C{{H}_{3}}-HC=C(Br)-{{C}_{2}}{{H}_{5}}\]
Cis-trans isomerism are shown in a compound when there is a difference in the orientation of the same two groups.
When the two same groups are on the same side of the carbon-carbon bond, the compound exhibits cis isomerism, on the other hand if the same groups lie on the opposite to each other of the C-C bond, they exhibit trans isomerism.
The structure of formed compound is given as:
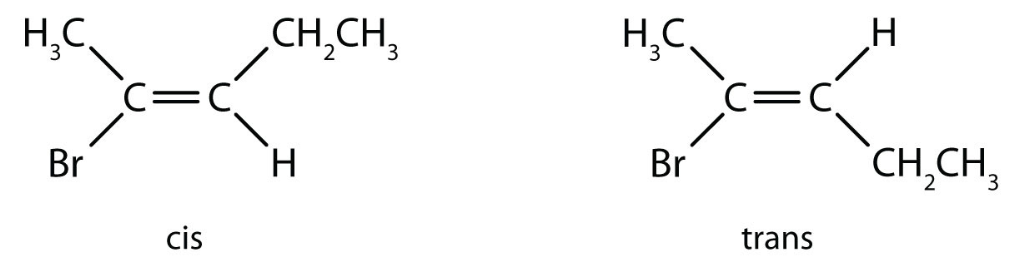
(E) (Z)

(E) (Z)
Therefore, there are 2 structural isomers. Each of these will lead to Z & E configurational isomers. i.e. total 4.
So, the correct option is (b).
Note: Cis-trans are a type of geometric isomerism which exists when there is a restriction in rotation of a molecule and there are two nonidentical groups on each doubly bonded carbon atom. Cis-trans isomerism are also exhibited by cyclic compounds.
Complete step by step solution:
Hydrogen bromide and 2-pentyne react together to form 3-bromopent-2-yne and 2-bromopent-2-yne as a final product. The reaction is given as:
\[2C{{H}_{3}}-C\equiv C-{{C}_{2}}{{H}_{5}}+2HBr\to C{{H}_{3}}-(Br)C=CH-{{C}_{2}}{{H}_{5}}+C{{H}_{3}}-HC=C(Br)-{{C}_{2}}{{H}_{5}}\]
Cis-trans isomerism are shown in a compound when there is a difference in the orientation of the same two groups.
When the two same groups are on the same side of the carbon-carbon bond, the compound exhibits cis isomerism, on the other hand if the same groups lie on the opposite to each other of the C-C bond, they exhibit trans isomerism.
The structure of formed compound is given as:
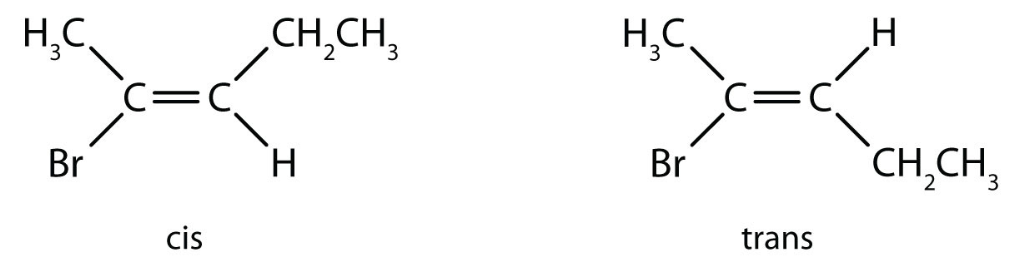
(E) (Z)

(E) (Z)
Therefore, there are 2 structural isomers. Each of these will lead to Z & E configurational isomers. i.e. total 4.
So, the correct option is (b).
Note: Cis-trans are a type of geometric isomerism which exists when there is a restriction in rotation of a molecule and there are two nonidentical groups on each doubly bonded carbon atom. Cis-trans isomerism are also exhibited by cyclic compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




