
The number of $s{p^3}$ hybridized carbon atoms in ascorbic acid and dehydroascorbic acid are respectively:
A.3,3
B.3,5
C.5,3
D.4,5
Answer
585.6k+ views
Hint: Ascorbic acid basically belongs to the monosaccharide family and has a chemical formula ${C_6}{H_8}{O_6}$ . It is basically vitamin C whereas dehydroascorbic acid is an oxidized form of ascorbic acid with chemical formula ${C_6}{H_6}{O_6}$ .
Complete step by step answer:
Ascorbic acid is basically vitamin C that should be obtained I the diet as it cannot be produced by humans. It is present in citrus fruits, strawberries, broccoli, kiwifruit, sprouts etc. whereas dehydroascorbic acid is an oxidized form of ascorbic acid. It is imported into the endoplasmic reticulum of the cell via glucose.
Now, hybridization is defined as the concept of intermixing of two atomic orbitals with same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics. Furthermore, in case of $s{p^3}$ hybridization, one’s’ orbital and 3 ‘p’ orbitals belong to the same shell of an atom mix together to form four new equivalent orbitals. It is also known as tetrahedral hybridization.
Now, the number of $s{p^3}$ hybridized carbon atoms in ascorbic acid and dehydroascorbic acid are 3,3 respectively. Let’s have a look at their structures:
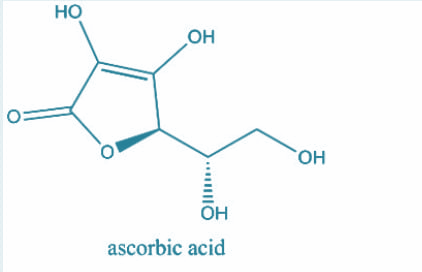
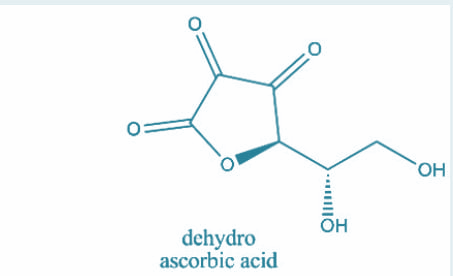
Hence, option A is correct.
Note:
Ascorbic acid is used in the treatment of scurvy, in the formation of collagen fibers in connective tissues, fibrous tissues, bones and teeth. It fights against the bacterial infections and is used to prevent the transfer of HIV from mothers to babies. Moreover, it is also used in the treatment of stomach ulcers and to prevent gallbladder disease.
Complete step by step answer:
Ascorbic acid is basically vitamin C that should be obtained I the diet as it cannot be produced by humans. It is present in citrus fruits, strawberries, broccoli, kiwifruit, sprouts etc. whereas dehydroascorbic acid is an oxidized form of ascorbic acid. It is imported into the endoplasmic reticulum of the cell via glucose.
Now, hybridization is defined as the concept of intermixing of two atomic orbitals with same energy levels to give a degenerated new type of orbitals. This intermixing is based on quantum mechanics. Furthermore, in case of $s{p^3}$ hybridization, one’s’ orbital and 3 ‘p’ orbitals belong to the same shell of an atom mix together to form four new equivalent orbitals. It is also known as tetrahedral hybridization.
Now, the number of $s{p^3}$ hybridized carbon atoms in ascorbic acid and dehydroascorbic acid are 3,3 respectively. Let’s have a look at their structures:


Hence, option A is correct.
Note:
Ascorbic acid is used in the treatment of scurvy, in the formation of collagen fibers in connective tissues, fibrous tissues, bones and teeth. It fights against the bacterial infections and is used to prevent the transfer of HIV from mothers to babies. Moreover, it is also used in the treatment of stomach ulcers and to prevent gallbladder disease.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




